Volume 10, Number 10—October 2004
Dispatch
Campylobacteriosis, Eastern Townships, Québec
Abstract
Independent risk factors for campylobacteriosis (eating raw, rare, or undercooked poultry; consuming raw milk or raw milk products; and eating chicken or turkey in a commercial establishment) account for <50% of cases in Québec. Substantial regional and seasonal variations in campylobacteriosis were not correlated with campylobacter in chickens and suggested environmental sources of infection, such as drinking water.
Published case-control studies provide conflicting results regarding the risk factors for sporadic campylobacteriosis. Poultry is commonly considered the principal source, and in some studies, was implicated in 50% to 70% of endemic cases (1,2). Campylobacter have been frequently cultured from poultry during processing (47%–82%) and retail distribution (23%–62%) (3–6). However, some studies observed no significant risk associated with eating chicken (7,8); in other studies, this factor was actually protective (9,10). We describe a prospective case-control study of domestically acquired Campylobacter infections combined with a prevalence study of Campylobacter spp. in whole retail chickens purchased in the Eastern Townships, Québec.
The Eastern Townships comprise seven counties and total ≈300,000 inhabitants. Hospital microbiology laboratories routinely report all Campylobacter enteritis cases to the regional public health department. All the laboratories in the study region, except in Granit County, routinely evaluated stool specimens for Campylobacter by using comparable standard methods for isolation and identification (Karmali or Skirrow media incubated for 72 h at 42°C in a microaerobic atmosphere). Granit County’s laboratory sent stool specimens for Campylobacter culture to our hospital microbiology laboratory on special medical request only. Incidence rates of campylobacteriosis in the Eastern Townships and Québec Province were calculated with demographic and reportable diseases data from provincial registers.
All cases reported from July 1, 2000, through September 30, 2001, were eligible. Case-patients were excluded if the infection was acquired outside Québec (i.e., travel abroad during the 10-day period before the onset of symptoms) or if the interval between the onset of symptoms and reporting was >6 weeks. All investigations were conducted within 2 weeks of reporting. For participants with infections reported on multiple occasions during the study period, the first episode of infection was considered. The median interval from the onset of symptoms to the interview of the cases was 13 days (range 5–56 days; 90th percentile, 23 days).
Each case was matched for sex and age group (<1, 1–4, 5–14, 15–34, 35–64, and >65 years) to two controls living in the Eastern Townships, who were identified through random digit dialing. Patients and controls were interviewed by telephone with a structured questionnaire to capture demographic and clinical data, travel history, food history, water consumption, recreational water activity, animal contacts, and other illness during the 10 days before the onset of symptoms. Controls had to be interviewed within 3 weeks of the patient and were excluded if they could not be reached after three telephone calls; had fever, abdominal pain, nausea, vomiting, diarrhea, or bloody stools; traveled abroad during the 10-day period before the patient’s onset of symptoms; or refused to participate. Controls did not have stool samples tested for Campylobacter. A surrogate parent was interviewed when the patient or control was a child <14 years of age. The interviewers were not blinded to the patient or control status of study participants.
Risk factors for campylobacteriosis were evaluated by conditional logistic regression for matched data adjusted for the county of residency. All risk factors with p < 0.05 by univariate analysis were included in a multivariate, conditional, logistic regression, stepwise selection model for matched data. All statistical analyses were performed using SAS version 6.1 (SAS, Cary, NC).
During the study, four fresh, eviscerated whole chickens were bought weekly in different counties (one chicken per store); for each county, the number of chickens sampled monthly was proportional to the population. Retail chickens sold in the Eastern Townships are produced by multiple companies based elsewhere in Québec Province.
The chickens were stored at 4°C overnight and washed vigorously with 250 mL of nutrient broth. The broth was filtered through cheesecloth and centrifuged at 16,300 x g for 15 min. The sediment was suspended in 5 mL of brucella broth; 100 mL of Park and Sanders’ selective enrichment broth with 0.5 mL of Supplement A (0.2% vancomycin and 0.2% trimethoprim lactate) and 5 mL of Supplement B (0.064% sodium cefoperazone in brucella broth) (11) were added to the suspension, gently mixed, and incubated under microaerobic atmosphere at 37°C for 4 h, then at 42°C for 48 h. Three loopfuls (0.05 mL) of the suspension were plated on Karmali agar and incubated at 42°C for 48 h under microaerobic conditions. Isolates of Campylobacter were identified to the species level by routine phenotypic methods.
From July 2000 through October 2001, a total of 201 cases of campylobacteriosis were reported, of which 43 were excluded: 18 patients acquired their infection outside Québec, 18 resided outside the Eastern Townships, 6 could not be interviewed within 6 weeks after the onset of symptoms, and patient declined to participate. All but two patients were matched to two controls each; consequently, the final dataset comprised 158 cases and 314 controls. Cases and controls were well-distributed across the seven counties, except in Val St-François, which represented 15% of cases and 7% of controls (data not shown).
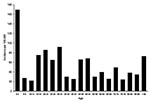
During the study period, the mean crude incidence of campylobacteriosis was 63.1/100,000 in the Eastern Townships, compared to 44.5/100,000 in the remainder of Québec Province (p < 0.0001). Most cases occurred during July, August, and September (Figure 1). The median age of the case-patients was 31 years (range 11 days to 91 years). The incidence of campylobacteriosis varied considerably by age (Figure 2), with the highest rates among children 0–4 years of age (169.2/100,000) and young adults 15–34 years of age (mean = 79.4/100,000). Overall, 64 (40.5%) participants were female.
The rates varied from 38.3/100,000 in Memphrémagog to 113.5/100,000 in Asbestos (excluding Granit, where case ascertainment was different); these interregional differences persisted after stratification for age (Table 1). The risk of campylobacteriosis was 2.4-fold higher in Asbestos (p = 0.0001) and 1.3-fold higher in Val St-François (p = 0.04) than elsewhere in the Eastern Townships.
Among 41 exposure factors evaluated by univariate conditional logistic, four achieved p values < 0.01 (Table 2). Two were associated with poultry: eating raw, rare, or undercooked poultry (p = 0.003) and eating turkey or chicken in a restaurant, a fast food establishment, or restaurant with a buffet (p = 0.004). Two were associated with other exposures: consuming raw milk or raw milk products (p = 0.0001) and professional exposure to animals or a contact with farm or zoo animals (p = 0.0003). No other activity related to consuming or handling poultry appeared related to infection (Table 2).
Conditional multivariate analysis adjusted for the county of residency resolved only three independent risk factors: raw, rare, or undercooked poultry (odds ratio [OR] 5.00, 95% confidence interval [CI ] 1.79–13.98, p = 0.002), raw milk or raw milk products (OR 3.67, 95% CI 1.95–6.90, p = 0.0001), and turkey or chicken eaten in a restaurant, a fast food or a buffet (OR 1.96, 95% CI 1.24–3.11, p = 0.004). These factors accounted for 8%, 18%, and 20% of cases, respectively.
A total of 177 chickens from 58 different food stores were cultured (median per month, 16; range 8–20). Campylobacter spp. were cultured from 41 (23%) (37 C. jejuni; 4 C. coli). The prevalence of Campylobacter was low from November 2000 to July 2001 inclusively, with 0–2 positive chickens (0%–25%) per month (Figure 1) but increased sharply in August, September, and October 2001, with rates reaching 69%, 55%, and 56%, respectively. The number of locally acquired Campylobacter enteritis in humans peaked at 16 cases in July 2001 (i.e., 1 month before the peak of chicken contamination) and then decreased to 11, 3, and 3 cases in August, September, and October 2001, respectively. Further, we analyzed data for each county separately and found no geographic correlation between campylobacteriosis in humans and Campylobacter in chickens (p = 0.42). Thus, although chicken consumption is an important risk factor for Campylobacter enteritis, it does not explain either the seasonal or regional variations in the incidence of sporadic cases of campylobacteriosis in humans.
Exposures to poultry account for fewer than half the episodes of sporadic Campylobacter infection. Substantial seasonal and interregional variations suggest environmental sources of infection. In the univariate analysis, drinking tap water at home or at work tended to be associated with an increased risk for infection (OR 1.90, p = 0.03), and in a subanalysis of cases in Asbestos County, which had the highest incidence, drinking tap water from a deep well at home was the only risk factor identified (53% of cases compared to 23% of controls; OR 3.83, p = 0.06 by univariate analysis and OR 3.96, p = 0.06 after adjusting for age group and sex). A recent case-control study (12) identified drinking water that was not disinfected as an independent risk factor for campylobacteriosis, with an etiologic fraction of 26%. These results are consistent with the hypothesis that the waterborne route of infection may be the common underlying pathway linking infection in humans, poultry, other domestic animals, and wild birds.
In waterborne outbreaks associated with Campylobacter, fecal contamination of the drinking water source has been traced to runoff of surface water after rain or to leakage from a sewage line into an adjacent drinking water pipe (13–15). Since a few hundred viable organisms represent an infectious dose, even apparently low levels of contamination could result in infection. The true importance of drinking water as a source of sporadic infection in humans may have been underestimated in the past and should be investigated in future studies.
Dr. Michaud is a medical microbiologist and an infectious diseases specialist at the Centre Hospitalier Universitaire de Sherbrooke and an adjunct professor at the Faculté de Médecine de l’Université de Sherbrooke, Québec, Canada. Her primary research interests are the clinical and molecular epidemiology of C. jejuni enteritis.
Acknowledgments
We thank Diane Dion, Danielle Proulx, Linda Billard, and Mélanie Proulx for data collection; Reno Proulx for designing the random digit dial system; and Bruno Maynard for his appreciable help in purchasing chickens.
Financial support was provided by Ministère de la Santé et des Services Sociaux du Québec, the Régie Régionale de la Santé et des Services Sociaux de l'Estrie, and the Centre de Recherche Clinique du Centre Hospitalier Universitaire de Sherbrooke.
References
- Neal KR, Slack RC. The autumn peak in Campylobacter gastroenteritis. Are the risk factors the same for travel- and UK-acquired campylobacter infections? J Public Health Med. 1995;17:98–102.PubMedGoogle Scholar
- Harris NV, Weiss NS, Nolan CM. The role of poultry and meats in the etiology of Campylobacter jejuni/coli enteritis. Am J Public Health. 1986;76:407–11. DOIPubMedGoogle Scholar
- Park CE, Stankiewicz ZK, Lovett J, Hunt J. Incidence of Campylobacter jejuni in fresh eviscerated whole market chickens. Can J Microbiol. 1981;27:841–2. DOIPubMedGoogle Scholar
- Harris NV, Thompson D, Martin DC, Nolan CM. A survey of Campylobacter and other bacterial contaminants of pre- market chicken and retail poultry and meats, King County, Washington. Am J Public Health. 1986;76:401–6. DOIPubMedGoogle Scholar
- Bryan FL, Doyle MP. Health risks and consequences of Salmonella and Campylobacter jejuni in raw poultry. J Food Prot. 1995;58:326–44.
- Prescott JF, Gellner OS. Intestinal carriage of Campylobacter jejuni and Salmonella by chicken flocks at slaughter. Can J Comp Med. 1984;48:329–31.PubMedGoogle Scholar
- Rodrigues LC, Cowden JM, Wheeler JG, Sethi D, Wall PG, Cumberland P, The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol Infect. 2001;127:185–93. DOIPubMedGoogle Scholar
- Effler P, Ieong MC, Kimura A, Nakata M, Burr R, Cremer E, Sporadic Campylobacter jejuni infections in Hawaii: associations with prior antibiotic use and commercially prepared chicken. J Infect Dis. 2001;183:1152–5. DOIPubMedGoogle Scholar
- Adak GK, Cowden JM, Nicholas S, Evans HS. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of campylobacter infection. Epidemiol Infect. 1995;115:15–22. DOIPubMedGoogle Scholar
- Eberhart-Phillips J, Walker N, Garrett N, Bell D, Sinclair D, Rainger W, Campylobacteriosis in New Zealand: results of a case-control study. J Epidemiol Community Health. 1997;51:686–91. DOIPubMedGoogle Scholar
- Sanders G. Isolation of Campylobacter from food. Laboratory Procedure MFLP-46. Ottawa, Canada: Health Protection Branch; 1998.
- Kapperud G, Espeland G, Wahl E, Walde A, Herikstad H, Gustavsen S, Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. Am J Epidemiol. 2003;158:234–42. DOIPubMedGoogle Scholar
- Waterborne outbreak of gastroenteritis associated with a contaminated municipal water supply, Walkerton, Ontario, May–June 2000. Can Commun Dis Rep. 2000;26:170–3.PubMedGoogle Scholar
- Hanninen ML, Haajanen H, Pummi T, Wermundsen K, Katila ML, Sarkkinen H, Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl Environ Microbiol. 2003;69:1391–6. DOIPubMedGoogle Scholar
- Koenraad PMFJ, Rombouts FM, Notermans SHW. Epidemiological aspects of thermophilic Campylobacter in water-related environments: a review. Water Environ Res. 1997;69:52–63. DOIGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 10, Number 10—October 2004
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Sophie Michaud, Department of Microbiology and Infectious Diseases, Faculté de Médecine de l'Université de Sherbrooke, 3001, 12e Avenue Nord, Sherbrooke, Québec J1H 5N4; fax: 819-564-5392
Top