Volume 11, Number 7—July 2005
Dispatch
SARS Coronavirus Detection Methods
Figure
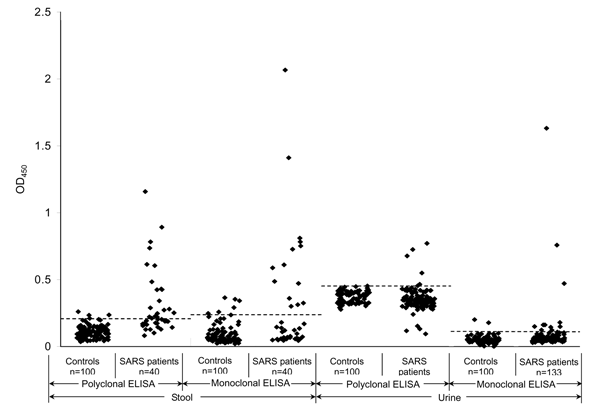
Figure. . Evaluation of polyclonal and monoclonal antibody–based enzyme-linked immunosorbent assays (ELISAs) for detecting nucleocapsid protein in fecal and urine specimens. The dashed horizontal lines represent the corresponding cutoff optical density values at 450 nm (OD450). SARS, severe acute respiratory syndrome.
Page created: April 23, 2012
Page updated: April 23, 2012
Page reviewed: April 23, 2012
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.