Volume 12, Number 2—February 2006
Dispatch
Waterborne Toxoplasmosis, Brazil, from Field to Gene
Abstract
Water was the suspected vehicle of Toxoplasma gondii dissemination in a toxoplasmosis outbreak in Brazil. A case-control study and geographic mapping of cases were performed. T. gondii was isolated directly from the implicated water and genotyped as SAG 2 type I.
Water has been considered an important vehicle for disseminating human toxoplasmosis in outbreaks (1,2) and in endemic settings in Brazil (3). We investigated a large toxoplasmosis outbreak in which the exposure to known sources of Toxoplasma gondii infection was assessed. We found that unfiltered, municipally treated water was the epidemiologically implicated source of infection for this outbreak. Isolation, polymerase chain reaction (PCR) detection, and genotyping of T. gondii from the implicated water source were demonstrated.
In November 2001, in Santa Isabel do Ivai, (southern state of Paraná), a local physician requested serologic tests to diagnose dengue, mononucleosis, cytomegalovirus infection, hepatitis, and toxoplasmosis in 2 persons in whom fever, headache, and myalgias had developed. Positive results were obtained for anti–T. gondii immunoglobulin M (IgM) and IgG only. Through the end of 2001, 294 similar cases, which were serologically confirmed as toxoplasmosis, were reported to health authorities in the same area.
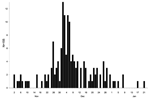
The outbreak peaked between November 2001 and January 2002 (Figure 1). Symptoms were reported by 155 persons; the main symptoms were headache (n = 135), fever (n = 128), malaise (n = 128), myalgia (n = 124), lymphadenitis (n = 117), anorexia (n = 107), arthralgia (n = 95), night sweats (n = 83), vomiting (n = 60), and rash (n = 11). The duration and magnitude of the epidemic curve could have been influenced by the intensity of media reporting at specific times, which led to people seeking toxoplasmosis testing.
Case-patients were located by active contacting of and passive reporting from local physicians, and media campaigns (television, radio, and newspapers). A matched case-control study was conducted from January 15 to February 2, 2002. Acute cases were defined by standard serologic criteria (4) and were selected from a list of volunteers. A total of 2,884 of 6,771 persons living in the urban area of the city volunteered to be serologically tested. A total of 426 (11.5%) persons had anti–T. gondii IgM and IgG antibodies; 1,255 (51%) were positive only for IgG antibodies. Of 426 persons who had anti–T. gondii IgM and IgG antibodies, 176 met the case definition; of these, 156 (89%) participated in the case-control study. Sex and age matched controls (±5 years, n = 220) were selected from the same group of volunteer who were asymptomatic and seronegative for T. gondii.
Serum samples from case-patients and controls were tested for anti–T. gondii IgM and IgG antibodies by the Central Laboratory of the Paraná State by using 3 different commercially available enzyme-linked immunosorbent assays (ELISAs) because it was not possible for a single vendor to provide the number of required kits. Fifty percent of the case serum samples (78 samples of 156 participants) were randomly retested in a toxoplasmosis serology reference laboratory, Laboratory of Protozoology at the Tropical Medicine Institute of São Paulo. Five (6.4%) IgM- and IgG-positive serum samples, tested previously with 1 of the commercial kits, showed very low IgG avidity when tested by this laboratory. All the other serum sample test results were confirmed by testing conducted in this laboratory.
Of the 156 participants, 138 (88%) lived in the area served by reservoir A and 17 individuals lived in area served by reservoir B (Figure 2); 1 person had a private well. Table 1 shows the univariate analysis results and Table 2 shows the multivariate analysis results. Case-patients were significantly more likely than controls to drink water supplied by municipal reservoir A than reservoir B, as well as to eat commercial ice cream than not. The 4 case-patients that reported not drinking water from reservoir A, however, reported eating ice cream. The frequency of eating ice cream among the persons who drank water from the reservoir A was 32%.
The environmental investigation included mapping the city water supply system which is served by 2 municipal tank reservoirs (reservoir A and reservoir B) that both receive water from underground, protected deep wells. Both reservoirs are tanks with 150,000 L storage capacity. Case distribution showed a clustering in the central area served by reservoir A (Figure 2).
Because the environmental investigations and the case-control study started in parallel on January 9, 2002, and the outbreak had peaked (Figure 1), the chances of detecting parasites in the municipally distributed water were theoretically low. To increase the chances of detecting the parasite in water, household tanks that had water that had been distributed during the outbreak peak were identified. These tanks could be investigated in municipal schools that stopped water use due to summer vacations from December 17, 2001, to the end of January 2002. Despite the risk from eating ice cream (Table 2), no ice cream made during the outbreak period was available for laboratory testing. The ice cream was prepared locally in small batches with water from reservoir A.
We identified 4 schools that had water in their household tanks that had been distributed by reservoir A during the peak of the outbreak. Approximately 4,650 L of water collected from these tanks was filtered through 56 fluoropore membrane filters (Millipore Billerica, MA, USA). We retrieved 19 liters of water concentrated to 60 mL by centrifugation (600 × g 30 min 4°C). The membrane filters were divided into 3 equal sets. One set remained in Brazil (Universidade Estadual do Norte Fluminense Darcy Ribeiro) for bioassays in T. gondii–seronegative chickens and further genotyping. One set was sent to the US Department of Agriculture for bioassays in T. gondii–seronegative pigs and cats, and 1 was sent to the Centers for Disease Control and Prevention for PCR analysis. Chickens and pigs were fed with membrane filters and their serum samples tested by ELISA and or modified agglutination test (5) until seroconversion. The seropositive animal organs were examined for T. gondii (6). Control animals were fed with noncontaminated membrane filters. Water samples from the 4 schools' household tanks were positive for T. gondii by at least 1 assay method. Parasites were found in the lungs of mice injected with brain and heart tissue of seropositive chickens. Cats fed pig tissues shed T. gondii oocysts after 4–5 days. Oocysts from cat feces were injected into mice, which died of acute toxoplasmosis. Viable T. gondii was recovered in mice after subpassage as verified by optical microscopy. The nested amplification of SAG 2 followed by restriction fragment length polymorphism identified type I T. gondii from chickens and pigs (7).
DNA extraction from fluoropore membranes was performed with the FastDNA extraction method (Qbiogene, Irvine, CA, USA), by using a procedure previously published (8), and PCR was performed on extracted DNA by using primers Toxo B22 and B23 (9). PCR from DNA extracted directly from the fluoropore membranes was tested blindly by 2 persons on 3 aliquots extracted individually from each membrane filter. The correct size fragment of 115 bp from B1 T. gondii gene was amplified from each DNA aliquot extracted from membranes used to process water from 3 of the implicated tanks.
Our investigation determined that this toxoplasmosis outbreak was associated with consumption of contaminated water, or ice cream prepared with contaminated water, during the outbreak peak. The main factor leading to contamination of reservoir A was the vulnerability to infiltration due to its precarious state of conservation. We propose that reservoir A was contaminated with T. gondii oocysts because 1) a female cat living in the reservoir A area delivered 3 kittens in early October 2001; 2) the kittens lived on the top of the tank reservoir; and 3) the kittens were most likely weaned by the first week of November. However, it was not possible to confirm T. gondii in the kittens because we were not able to catch them. The reservoir shelter roof tiles were removed and not replaced until the end of heavy summer rains. From November 4 to December 12, the daily rainfall varied from 27 mm to 72 mm. Reservoir A, constructed in the 1940s, had cracks that were unprotected from rain water, which were likely contaminated with cat feces. These factors could have been enhanced by the lack of filtration and flocculation processes as part of the water treatment. Additionally, the level of chlorination used to treat water in municipal systems is inadequate to eliminate T gondii oocysts (10).
Of the 408 case-patients examined for ophthalmologic conditions through February of 2002 who were Toxoplasma IgM and IgG positive, 10% had ocular lesions; however, only 4.4% had necrotizing retinal lesions (11). The frequency of symptoms observed in this study may be associated with the dose and virulence of organisms ingested since parasites of genotype I, which are of high virulence (12,13), were isolated from the water implicated in the outbreak. These data are consistent with other studies also showing SAG-2 type I parasites isolated from the environment from different geographic areas in Brazil (14), including in the outbreak area (15). Demonstration of the parasite in the outbreak implicated water was decisive in the closing of reservoir A and the construction of a new municipal reservoir.
Mr de Moura is a registered nurse and epidemiologist and member of the Training Programs in Epidemiology and Public Health Interventions Network (TEPHINET).
Acknowledgments
We thank Jarbas Barbosa da Silva Junior, Maria Regina Fernandes de Oliveira, Ana Eliza Mazzotini, Antônio Moreno, Marcos Cardoso Marques, Sebastião Quiel, Benedito Souza, Cilso Gomes, Joel Luís da Silva, Fábio Guardalin, Gisela Janaina Marques, Kátia Sirlene Tavares, Nereu Henrique Mansano, Luciane Zappelini Daufenbach, Greice Madeleine Ilkeda do Carmo, Carmen Lúcia Muricy, Wildo Navegantes de Araújo, Alessandra Araújo Siqueira, Roberto Mello Dusi, Heitor Franco de Andrade Jr, Enrique Medina Acosta, Érica dos Santos Carvalho, Fernanda Santos Nascimento, Patricia Silva Santos, Daniele Seipel da Silva, Juliana Salgado Viana, Fernando César Lopes, James Maguire, and Mark Eberhard for their assistance with this article.
This study was supported by the Ministry of Health of Brazil, CNPq PRONEX (2704) and Universidade Estadual do Norte Fluminense Darcy Ribeiro.
The institutional review board at Ministry of Health approved submission of this manuscript for publication.
References
- Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N Engl J Med. 1982;307:666–9. DOIPubMedGoogle Scholar
- Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet. 1997;350:173–7. DOIPubMedGoogle Scholar
- Bahia-Oliveira LMG, Jones JL, Azevedo-Silva J, Oréfice F, Crespo C, Addiss D. Highly endemic waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis. 2003;9:55–62.PubMedGoogle Scholar
- Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant, 5th ed. Philadelphia: WB Saunders; 2001. p. 205–346.
- Dubey JP, Ruff MD, Camargo ME, Shen SK, Wilkins GL, Kwok OC, Serologic and parasitologic responses of domestic chickens after oral inoculation with Toxoplasma gondii oocysts. Am J Vet Res. 1993;54:1668–72.PubMedGoogle Scholar
- Dubey JP. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet Parasitol. 1998;74:75–7. DOIPubMedGoogle Scholar
- Lehmann T, Blackstonn CR, Parmley SF, Remington JS, Dubey JP. Strain typing of Toxoplasma gondii: comparison of antigen-coding and house keeping genes. J Parasitol. 2000;86:960–71.PubMedGoogle Scholar
- da Silva AJ, Bornay-Llinares FJ, Moura IN, Slemenda SB, Tuttle JL, Pieniazek NJ. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol Diagn. 1999;4:57–64. DOIPubMedGoogle Scholar
- Bretagne S, Costa JM, Vidaud M, Tran J, Nhieu V, Fleury-Feith J. Detection of Toxoplasma gondii by competitive DNA amplification of bronchoalveolar lavage samples. J Infect Dis. 1993;168:1585–8. DOIPubMedGoogle Scholar
- Aramini JJ, Stephen C, Dubey JP, Engelstoft C, Schwantje H, Ribble CS. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol Infect. 1999;122:305–15. DOIPubMedGoogle Scholar
- Silveira CAM. A maior Epidemia do mundo. In: Silveira CAM, editor. Toxoplasmose Dúvidas e Controvérsias, 1st ed. Erechim: Edifapes; 2002. p. 79–82.
- Howe DK, Summers BC, Sibley LD. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii. Infect Immun. 1996;64:5193–8.PubMedGoogle Scholar
- Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–42. DOIPubMedGoogle Scholar
- Dubey JP, Graham DH, Seipel DS, Lehmann T, Bahia-Oliveira LMG. Toxoplasma gondii isolates of free-ranging chickens from Rio de Janeiro, Brazil: mouse mortality, genotype, and oocyst shedding by cats. J Parasitol. 2003;89:851–3. DOIPubMedGoogle Scholar
- Dubey JP, Navarro IT, Sreekumar C, Dahl E, Freire RL, Kawabata HH, Toxoplasma gondii infection in cats from Paraná Brazil: seroprevalence, tissue distribution, and biologic and genetic characterization of isolates. J Parasitol. 2004;90:721–6. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1These authors contributed equally to this study.
Table of Contents – Volume 12, Number 2—February 2006
| EID Search Options |
|---|
|
|
|
|
|
|