Volume 12, Number 2—February 2006
Dispatch
Evaluation of a Direct, Rapid Immunohistochemical Test for Rabies Diagnosis
Abstract
A direct rapid immunohistochemical test (dRIT) was evaluated under field and laboratory conditions to detect rabies virus antigen in frozen and glycerol-preserved field brain samples from northwestern Tanzania. Compared to the direct fluorescent antibody test, the traditional standard in rabies diagnosis, the dRIT was 100% sensitive and specific.
In much of the developing world, rabies surveillance and diagnosis in domestic and wild animals are severely constrained. High ambient temperatures hinder the collection and preservation of fresh specimens. The use of the direct fluorescent-antibody assay (DFA), the traditional standard in rabies diagnosis (1,2), is limited by the costs of acquiring and maintaining a fluorescent microscope. Difficulties in obtaining diagnostic results from field material have led to widespread underreporting of disease.
Consequently, the true public health impact of rabies has been greatly underestimated (3–5), and political commitment for its control has been lacking. Moreover, the absence of a confirmatory test can result in the inappropriate management of animal bite injuries, with human deaths a potential consequence of delays in rabies postexposure prophylaxis (PEP) and unnecessary administration of PEP. The latter is a particular concern, given the scarcity and costs of human rabies vaccines and immunoglobulin in many parts of the world.
A rapid immunohistochemical test (RIT) to detect rabies virus (RABV) antigen has been developed in the Rabies Section of the Centers for Disease Control and Prevention (CDC) by incorporating various components of existing immunoperoxidase techniques (6). Like the DFA, the RIT is performed on brain touch impressions, but the product of the reaction can be observed by light microscopy, and RABV antigen appears as magenta inclusions against a blue neuronal background. The test recognizes all genotype 1 variants of RABV examined to date and all representative lyssaviruses. Modifications of a former indirect test have led to a direct test (dRIT) that uses a cocktail of highly concentrated and purified biotinylated anti-nucleocapsid monoclonal antibodies produced in vitro in a direct staining approach and allows a diagnosis to be made in <1 hour. For the routine diagnosis of rabies, glycerol saline is a convenient preservative in situations in which refrigeration or freezing facilities are not promptly available (7).
We report findings of a preliminary study to evaluate the dRIT, comparing results of the dRIT carried out under field conditions in Tanzania with the dRIT and DFA performed at CDC. The objectives were to validate the dRIT as a field test for rabies surveillance and evaluate the dRIT on glycerol-preserved field samples.
Brain stem samples from various animal species were obtained from December 2002 to September 2004 as a result of rabies surveillance operations established in the Mara, Mwanza, and Shinyanga regions of northwestern Tanzania. Some archived glycerolated specimens were also analyzed. Samples were collected by inserting a drinking-straw through the occipital foramen, according to World Health Organization recommendations (7) or by opening the skull.
Some specimens were frozen (–20°C). Other samples inside straws were placed into a solution of phosphate-buffered 50% glycerol and stored either at +4°C or at –20°C or kept at room temperature (25°C ± 5°C) for up to 4 months before refrigeration or freezing.
Samples were allocated to 4 groups, according to the method of preservation and whether the samples were tested in the field and at the CDC laboratory or at CDC only (Table 1). Group A samples were kept in glycerol solution for <15 months and washed in phosphate-buffered saline (PBS) before testing by dRIT in the field. They were then stored at –20°C for <5 months and retransferred into fresh glycerol for shipment. At CDC, the samples were kept in glycerol for <2 months and rewashed in PBS before retesting by both dRIT and DFA or DFA only. Group B samples were stored frozen for <6 months, processed by dRIT in the field, and placed into glycerol solution for shipment to CDC, where they were stored for 2 months before being washed in PBS and retested. Group C samples were preserved in glycerol solution for <60 months, shipped, and processed at CDC by dRIT and DFA without previous testing in the field. These samples were washed in PBS just before testing. Group D samples were stored at –20°C in the field for 2 to 24 months, shipped frozen, and tested at CDC by dRIT and DFA.
A qualitative assessment of the samples was made before testing. Five specimens at a time were stained by dRIT at ambient temperature as described below. Touch impressions were made on glass microscope slides as described (8). The slides were air-dried, fixed in 10% buffered formalin for 10 min, dip-rinsed in wash buffer PBS with 1% Tween 80 (TPBS), immersed in 3% hydrogen peroxide for 10 min, and dip-rinsed in fresh TPBS. After dipping, the excess buffer was shaken from the slides and blotted from the edges surrounding the impression. This treatment was repeated after each rinsing step. The slides were incubated in a humidity chamber (a cover on a moistened paper towel on an even surface) with the MAb cocktail for 10 min, dip-rinsed in TPBS, incubated with streptavidin-peroxidase complex (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD, USA) for 10 minutes and dipped in TPBS. A 3-amino-9-ethylcarbazole (AEC) stock solution was prepared by dissolving one 20-mg tablet AEC (Sigma-Aldrich Corp., St. Louis, MO, USA) in 4 mL N,N-dimethylformamide (Fisher Scientific International, Inc., Pittsburgh, PA, USA) and stored at 4°C. A working dilution was prepared by adding 1 mL AEC stock solution to 14 mL 0.1 mol/L acetate buffer (Polyscientific, Bay Shore, NY, USA) and 0.15 mL 3% hydrogen peroxide. The slides were incubated with the AEC peroxidase substrate for 10 min and dip-rinsed in distilled water. They were then counterstained with Gill's formation #2 hematoxylin (Fisher Scientific International) diluted 1:2 with distilled water for 2 min and dip-rinsed in distilled water. Finally, they were mounted with a water-soluble mounting medium (BioMeda Corp., Foster City, CA, USA) and examined by light microscopy (Leica Microsystems AG, Wetzlar, Germany) in Tanzania and Axioplan 2 (Carl Zeiss AG, Göttingen, Germany) at CDC at magnifications of ×200 to ×400. The same operator performed the dRIT in the field and at CDC. However, at CDC, identification numbers unknown to the operator were assigned. The DFA (FITC Anti-Rabies Monoclonal Globulin, Fujerebio Diagnostic Inc., Malvern, PA, USA) was performed in a blind manner by another operator as described (8) and read by fluorescent microscopy (Axioplan 2).
Confidence intervals for the sensitivity and specificity were calculated by using the exact binomial distribution (S-Plus, Insightful Corp., Seattle, WA, USA). Of 159 total samples tested, 59 specimens (37.1%) were positive for RABV antigen, and 100 (62.9%) were negative by dRIT, with 100% agreement between the tests, whether dRIT was performed in field conditions only, both in field and laboratory conditions, or in laboratory conditions only. Assuming that the DFA was 100% sensitive and specific, the dRIT was 100% sensitive (95% confidence interval [CI] 93.9%–100.0%) and 100% specific (95% CI 96.3%–100.0%). Table 2 shows the distribution of positive samples in the various animal species.
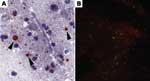
The sensitivities of the dRIT and DFA were comparable, regardless of the method of preservation. We have no evidence that storage times affected positivity because 34 (77.2%) of 44 samples stored in glycerol solution remained positive for up to 10 months before being tested in the field and retested at CDC after an interval of up to 6 months. Furthermore, RABV antigen was successfully detected in the sample that had been preserved in glycerol for the longest duration (15 months) before dRIT in the field, stored frozen for 3 months before shipment to CDC, and kept in glycerol for 2 months before being retested (Figure 1). Similarly, viral inclusions were detected in a sample stored frozen for 24 months, although the antigen distribution was sparse with both tests. Our data do not provide any unequivocal conclusions on test sensitivity with samples preserved in glycerol solution for >15 months because results from all 15 archived brains were negative. For these samples, the presence of antigen at the time of collection cannot be excluded.
Four of 10 (40.0%) deteriorated specimens were positive (Figure 2). Among the 6 brains with negative results, only 1 was suspected of containing rabies. The negative finding might have been caused by inadequate preservation, since the sample had been stored in glycerol solution at ambient temperature for up to 4 months before being refrigerated.
The dRIT showed a sensitivity and specificity equivalent to those of the DFA. The test is simple, requires no specialized equipment or infrastructure, and can be successfully performed on samples preserved in glycerol solution for 15 months or frozen for 24 months and in variable conditions of preservation. These qualities make it ideal for testing under field conditions and in developing countries. Although further laboratory and field evaluations are required, our results are promising and highlight the potential value of the dRIT for countries with limited diagnostic resources. First, this technique could greatly enhance epidemiologic surveillance in remote areas where rabies incidence data are difficult to obtain. Second, the test could improve the ability to respond to outbreaks with effective management decisions. Third, it could be extremely valuable in guiding decisions regarding rational use of rabies PEP.
Ms Lembo is a doctoral candidate at the Centre for Tropical Veterinary Medicine, University of Edinburgh. Her research interests include the epidemiology of viral diseases in developing country settings.
Acknowledgments
We are indebted to Tanzania National Parks, Tanzania Wildlife Research Institute, Ngorongoro Conservation Area Authority, Tanzania Commission for Science and Technology, and Tanzania Government ministries for permission to undertake research; the Tanzania National Parks Veterinary Unit, all members of the Viral Transmission Dynamics Project, the livestock officers of the Ministry of Water and Livestock Development in the Mara, Mwanza and Shinyanga regions, the Serengeti Lion and Cheetah Projects, the Frankfurt Zoological Society, the Veterinary Investigation Centres in Mwanza and Arusha, Mathias Magoto, Paul Tiringa, and Barbara Schachennuann-Suter for assistance with sample collection; Darren J. Shaw for assistance with the analysis; and Lesley Bell-Sakyi for providing valuable comments on the manuscript.
Rabies surveillance studies in northwestern Tanzania were supported by the joint National Institute of Health/National Science Foundation Ecology of Infectious Diseases Program under grant no. NSF/DEB0225453. Reagents were provided by the rabies section of CDC. Visits by T.L. to CDC were supported by the Royal (Dick) School of Veterinary Studies, University of Edinburgh, and the University of Edinburgh Development Trust. S.C. was supported by a Wellcome Trust Fellowship in Tropical Medicine for the early part of this work and by the UK Government Department for International Development Animal Health Programme.
References
- Goldwasser RA, Kissling RE. Fluorescent antibody staining of street and fixed rabies virus antigen. Proc Soc Exp Biol Med. 1958;98:219–23.PubMedGoogle Scholar
- Dean DJ, Abelseth MK, Atanasiu P. The fluorescent antibody test. In: Meslin F-X, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies, fourth edition. Geneva: World Health Organization; 1996. p. 66–79.
- Kitala PM, McDermott JJ, Kyule MN, Gathuma JM. Community-based active surveillance for rabies in Machakos District, Kenya. Prev Vet Med. 2000;44:73–85. DOIPubMedGoogle Scholar
- Cleaveland S, Fèvre EM, Kaare M, Coleman PG. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull World Health Organ. 2002;80:304–10.PubMedGoogle Scholar
- Coleman PG, Fèvre EM, Cleaveland S. Estimating the public health impact of rabies. Emerg Infect Dis. 2004;10:140–2.PubMedGoogle Scholar
- Niezgoda M, Rupprecht CE. Standard operating procedure for the direct rapid immunohistochemistry test for the detection of rabies virus antigen. National Laboratory Training Network Course. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. p. 1–16
- Barrat J. Simple technique for the collection and shipment of brain specimens for rabies diagnosis. In: Meslin F-X, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization; 1996. p. 425–32.
- Protocol for postmortem diagnosis of rabies in animals by direct fluorescent antibody testing. A minimum standard for rabies diagnosis in the United States [cited 2006 Jan 11]. Available from http://www.cdc.gov/ncidod/dvrd/rabies/Professional/publications/DFA_diagnosis/DFA_protocol-b.htm
Figures
Tables
Cite This ArticleTable of Contents – Volume 12, Number 2—February 2006
| EID Search Options |
|---|
|
|
|
|
|
|