Volume 12, Number 5—May 2006
Letter
Potential for Zoonotic Transmission of Brachyspira pilosicoli
To the Editor: Anaerobic intestinal spirochetes of the genus Brachyspira colonize the large intestine (1). Most Brachyspira species have a restricted host range, whereas Brachyspira (formerly Serpulina) pilosicoli colonizes a variety of animal and bird species and humans. B. pilosicoli is an important colonic pathogen of pigs and chickens (2). It occurs at high prevalence rates in humans in developing countries and in male homosexuals and HIV-positive persons in industrialized countries (3). Its potential as a human pathogen was emphasized after its identification in the bloodstream of a series of debilitated persons (4).
B. pilosicoli isolates from humans and other species have been used experimentally to colonize chicks, piglets, and mice (5–7). While these results indicate that the B. pilosicoli strains used lacked host-species specificity, few data exist on whether natural zoonotic spread of B. pilosicoli strains occurs. In 1 study that used pulsed-field gel electrophoresis (PFGE) to type isolates from Papua New Guinea, 2 dogs were colonized with B. pilosicoli isolates with the same PFGE types as those from villagers. However, the higher prevalence of colonization with B. pilosicoli in humans than dogs suggested that the dogs were infected with human isolates, probably through consumption of human feces (8).
Multilocus enzyme electrophoresis (MLEE) has been used to study variation in B. pilosicoli isolates; most studies have focused on isolates from only 1 or 2 host species (8–10). Generally, B. pilosicoli isolates are diverse, and a lack of linkage disequilibrium in the MLEE data for human isolates suggests that the species is recombinant (8).
We used MLEE to investigate relationships between 107 B. pilosicoli isolates of diverse geographic and host-species origins and the B. aalborgi type strain (NCTC 11492T). Isolates were selected on the basis of their diverse origins and availability in the Murdoch University culture collection. They originated from feces of 34 pigs, 19 chickens, 13 ducks, 1 rhea, 25 humans, and 4 dogs; from 7 human blood samples; and from 4 water sources frequented by waterfowl. Isolates originated from Australia, Canada, France, Italy, the Netherlands, Oman, Papua New Guinea, the United Kingdom, and the United States.
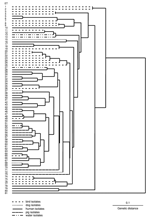
The MLEE method used was as previously described (8–10); the electrophoretic mobility of 15 constitutive enzymes was analyzed. Variations in electrophoretic mobility were interpreted as representing products of different alleles at each enzyme locus. Isolates with identical enzymatic profiles at 15 loci were grouped into an electrophoretic type (ET). Genetic distance between ETs was calculated as the proportions of loci at which dissimilar alleles occurred. PHYLIP version 3.51c (Phylogeny Inference Package, University of Washington, Seattle, WA, USA) was used to analyze data and generate a dendrogram by using the unweighted pair-group method with arithmetic mean clustering fusion strategy. Genetic diversity (h) was calculated for the number of ETs as (1 – Σpi2)(n/n – 1), where pi is the frequency of the indicated allele and n is the number of ETs.
B. pilosicoli isolates were divided into 80 ETs (mean 1.35 isolates per ET) (Figure). B. aalborgi NTCC 11492T was distinct in ET81. The B. pilosicoli isolates were diverse, with an h value of 0.41. Generally, they did not cluster according to host species of origin, and isolates from a given species were distributed throughout the dendrogram. Isolates from birds were more diverse than those from humans and pigs. Eight ETs contained multiple isolates, in each case from the same host species (either chickens or pigs). In 4 cases these originated from different countries: ET47 contained 2 Australian porcine isolates and 2 from the United States; ET53 contained 2 Australian porcine isolates and Scottish porcine type strain P43/6/78T; ET54 contained 2 Australian and 2 Canadian porcine isolates; ET65 contained 1 Dutch and 1 US chicken isolate.
Although human isolates did not share an ET with isolates from other species, they were frequently closely related, differing in 1 allele. This occurred with US and Australian pig isolates in ET47 and a human isolate from Oman in ET48; an Australian pig isolate in ET61 and a UK human isolate in ET62; an isolate from an Australian HIV-positive person in ET64, and 1 Dutch and 1 US chicken isolate in ET65; and a Papua New Guinea canine isolate in ET68 and a French human blood isolate in ET69.
The distribution continuum of isolates of diverse host species and geographic origin was consistent with a lack of species specificity and suggests that B. pilosicoli isolates naturally have the potential to be transmitted between species. Even should there be some unexpected species-specific barrier preventing "true" animal or bird isolates from colonizing humans, animals have been colonized by human isolates, and thus could act as a reservoir of these for subsequent retransmission to humans. The results suggest that zoonotic transfer of B. pilosicoli isolates likely occurs in nature, e.g., after exposure to infected animals or birds, their feces, or contaminated water.
References
- Stanton TB. Physiology of ruminal and intestinal spirochaetes. In: Hampson DJ, Stanton TB, editors. Intestinal spirochaetes in domestic animals and humans. Wallingford (UK): CAB International; 1997. p. 7–45.
- Hampson DJ, Duhamel GE. Porcine colonic spirochetosis/intestinal spirochetosis. In: Straw B, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of swine. 9th ed. Ames (IA): Iowa State University Press; 2006. p. 755–67.
- Hampson DJ. Intestinal spirochaetes. In: McIver CJ, editor. A compendium of laboratory diagnostic methods for common and unusual enteric pathogens–an Australian perspective. Sydney: ASM Publications; 2005. p. 101–8.
- Trott DJ, Jensen NS, Saint Girons I, Oxberry SL, Stanton TB, Lindquist D, Identification and characterization of Serpulina pilosicoli isolates from the blood of critically-ill patients. J Clin Microbiol. 1997;35:482–5.PubMedGoogle Scholar
- Trott DJ, McLaren AJ, Hampson DJ. Pathogenicity of human and porcine intestinal spirochaetes in day-old specific pathogen free chicks: an animal model of intestinal spirochetosis. Infect Immun. 1995;63:3705–10.PubMedGoogle Scholar
- Trott DJ, Huxtable CR, Hampson DJ. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infect Immun. 1996;64:4648–54.PubMedGoogle Scholar
- Sacco RE, Trampel DW, Wannemuehler MJ. Experimental infection of C3H mice with avian, porcine, or human isolates of Serpulina pilosicoli. Infect Immun. 1997;65:5349–53.PubMedGoogle Scholar
- Trott DJ, Mikosza ASJ, Combs BG, Oxberry SL, Hampson DJ. Population genetic analysis of Serpulina pilosicoli and its molecular epidemiology in villages in the Eastern Highlands of Papua New Guinea. Int J Syst Bacteriol. 1998;48:659–68. DOIPubMedGoogle Scholar
- Lee JI, Hampson DJ, Lymbery AJ, Harders SJ. The porcine intestinal spirochaetes: identification of new genetic groups. Vet Microbiol. 1993;34:273–85. DOIPubMedGoogle Scholar
- McLaren AJ, Trott DJ, Swayne DE, Oxberry SL, Hampson DJ. Genetic and phenotypic characterization of intestinal spirochetes colonizing chickens, and allocation of known pathogenic isolates to three distinct genetic groups. J Clin Microbiol. 1997;35:412–7.PubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 12, Number 5—May 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
David Hampson, School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch, Western Australia 6150, Australia; fax: 61-89-310-4144
Top