Volume 13, Number 12—December 2007
Dispatch
Parachlamydia spp. and Related Chlamydia-like Organisms and Bovine Abortion
Abstract
Chlamydophila abortus and Waddlia chondrophila cause abortion in ruminants. We investigated the role of Parachlamydia acanthamoebae in bovine abortion. Results of immunohistochemical analyses were positive in 30 (70%) of 43 placentas from which Chlamydia-like DNA was amplified, which supports the role of Parachlamydia spp. in bovine abortion.
Chlamydiae are implicated in a wide variety of clinically and economically important diseases in livestock and companion animals. Chlamydophila pecorum has been associated with abortion, conjunctivitis, encephalomyelitis, enteritis, pneumonia, and polyarthritis in ruminants, and Cp. abortus infection is the most common cause of abortion in sheep and goats (1). Cp. abortus also causes zoonotic infection in humans, which in pregnant women, can result in spontaneous abortion (2,3).
During the past decade, new Chlamydia-like organisms have been discovered and now emerge as possible public health threats. Simkania negevensis is considered a possible emerging agent of pneumonia (4), and evidence supports the role of Parachlamydia acanthamoebae as an agent of pneumonia (5,6). Waddlia chondrophila is another Chlamydia-like organism initially isolated from lung, liver, and other tissues of an aborted bovine fetus in the United States (7). This organism is now considered an abortigenic agent with a worldwide distribution in cattle, as shown by a recent report of Waddlia-related abortion in Germany (8).
The role of Chlamydia-like organisms in bovine abortion is further supported by results of a study of abortion in cattle in Graubünden, Switzerland (9). Analysis of placental specimens by PCR showed that 43 (18.3%) of 235 placentas contained DNA from Chlamydia-like organisms (9). Of these 43 specimens, 8 showed sequence similarity to P. acanthamoebae (95%–99%). Identification was not possible in the remaining 35 specimens because of their strong sequence similarity with uncultured chlamydial DNA sequences (Table). These 35 specimens were referred to as Chlamydia-like organisms. None of these 35 specimens was positive by immunohistochemical analysis with antibodies against Chlamydiaceae. This finding indicates that routine diagnostic approaches based on chlamydial lipopolysaccharide would not detect most Chlamydia-like infections (9). To confirm the role of these novel chlamydiae in bovine abortion, we analyzed these placental samples from cattle in Switzerland by using a new specific immunohistochemical protocol and transmission electron microscopy.
Formalin-fixed and paraffin-embedded placenta specimens were analyzed by using histopathologic and immunohistochemical techniques. Hematoxylin and eosin–stained histologic sections of all placenta specimens (n = 235) were examined for the type and degree of placentitis or vasculitis. Paraffin-embedded sections of specimens positive for Chlamydia-like organisms by 16S rRNA PCR (n = 43) were analyzed for Parachlamydia spp. and Waddlia by using specific mouse polyclonal antibodies as described (10). Optimization experiments for immunohistochemical analysis were performed by using infected amebal and infected HEp-2 cell pellets. Briefly, Acanthamoeba castellanii cultures were infected with P. acanthamoebae strain Hall coccus and W. chondrophila strain ATCC 1470. HEp-2 cell monolayers were infected with Cp. abortus strain S26/3. Uninfected cells were used as negative controls. Amebal and cell pellets were prepared as described (11). Optimization of the immunohistochemical protocol for experimentally infected amebal pellets showed the species specificity of mouse antibodies to P. acanthamoebae and W. chondrophila. We did not observe cross-reactivity of both antibodies with Cp. abortus–infected HEp-2 cell pellet (data not shown).
To test placental specimens, we used mouse polyclonal antibody against P. acanthamoebae and W. chondrophila at dilutions of 1:1,000 and 1:2,000, respectively. Antigen detection was performed with the ChemMate Detection Kit (Dako, Glostrup, Denmark) according to the manufacturer’s instructions. Briefly, paraffin-embedded sections were deparaffinated in xylene and rehydrated through graded ethanol to water. Antigen was detected by using repeated microwave heating (750 W for 10 min) in citrate buffer, pH 6.0 (Target Retrieval Solution, Dako). Specimens (slides) and primary antibodies were incubated for 1 hour. Negative and positive controls of each section were included as described (9).
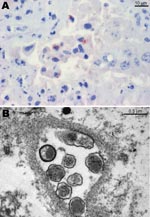
Histopathologic lesions such as purulent or necrotizing placentitis were observed in 149 (63.4%) of 235 specimens. Placentitis was observed in 5 of 8 specimens positive for P. acanthamoebae, and vasculitis was observed in 1 of 8 specimens (Table). Positive antigen labeling was observed in 6 of 8 specimens for Parachlamydia spp., but antigen labeling was negative in all specimens for Waddlia (Table). The Figure, panel A shows positive immunohistochemical labeling in 1 of these specimens. Among the 35 placentas positive by PCR for Chlamydia-like organisms other than P. acanthamoebae, 28 (82.3%) showed obvious purulent or necrotizing placentitis by histologic analysis. Four of the 28 specimens with placentitis also had vasculitis. A total of 24 (68.6%) of 35 specimens were positive when tested with antibody against P. acanthamoebae, and all 35 specimens were negative when tested with antibody against W. chondrophila.
Two placental specimens positive for Parachlamydia spp. by immunohistochemical analysis and 16S rRNA PCR were further investigated by transmission electron microscopy for ultrastructural evidence of Chlamydia-like organisms. Briefly, placental tissue specimens were fixed with glutaraldehyde and osmium tetroxide and embedded in Epon resin. Ultrathin sections (80 nm) were mounted on gold grids (Merck Eurolab, Dietlikon, Switzerland), contrasted with uranyl acetate dihydrate (Fluka, Buchs, Switzerland) and lead citrate (lead nitrate and tri-natrium dehydrate, Merck Eurolab), and analyzed with a Philips (Eindhoven, the Netherlands) CM10 electron microscope. Both placentas showed Chlamydia-like structures (Figure, panel B).
To our knowledge, this is the first description of Parachlamydia spp. in bovine abortion. The organism was detected by PCR (9) and within placental lesions by immunohistochemical analysis by using an antibody specific for Parachlamydia spp. and electron microscopy. All specimens were negative for Waddlia by immunohistochemical analysis. Isolation of Parachlamydia spp. from aborted bovines is necessary to confirm that this agent causes bovine abortion. Parachlamydia spp. may be involved in lower respiratory tract infections in humans (5,6) and may replicate within both pneumocytes (13) and human macrophages (14). Thus, caution should be taken when handling bovine abortion material because of the potential zoonotic risk.
Dr Borel is a researcher and pathologist at the Institute of Veterinary Pathology of the University of Zurich. Her research interests include the epidemiology and pathology of animal chlamydiosis and the role of obligate intracellular chlamydiae in ruminant abortions and their zoonotic potential to humans.
Acknowledgments
We thank Adam Polkinghorne for reviewing the manuscript; Ruedi Thoma for providing sample material; and the laboratory technical staff of the Institute of Veterinary Pathology, University of Zurich, and the Cantonal Laboratory of Veterinary Bacteriology, Chur, Switzerland, for assistance.
This study was supported by the State Secretary for Education and Research, Berne, Switzerland (project no. C05.0141) as part of the European Cooperation in the Field of Scientific and Technical Research Action 855. G.G. is supported by the Leenards Foundation through a career award, “Bourse leenards pour la relève académique en médecine clinique à Lausanne.”
References
- Aitken ID, Clarkson MJ, Linklater K. Enzootic abortion of ewes. Vet Rec. 1990;126:136–8.PubMedGoogle Scholar
- Pospischil A, Thoma R, Hilbe M, Grest P, Gebbers JO. Abortion in woman caused by caprine Chlamydophila abortus (Chlamydia psittaci serovar 1). Swiss Med Wkly. 2002;132:64–6.PubMedGoogle Scholar
- Longbottom D, Coulter LJ. Animal chlamydioses and zoonotic implications. J Comp Pathol. 2003;128:217–44. DOIPubMedGoogle Scholar
- Friedman MG, Dvoskin B, Kahane S. Infections with the Chlamydia-like microorganism Simkania negevensis, a possible emerging pathogen. Microbes Infect. 2003;5:1013–21. DOIPubMedGoogle Scholar
- Corsaro D, Greub G. Pathogenic potential of novel chlamydiae and diagnostic approaches to infections due to these obligate intracellular bacteria. Clin Microbiol Rev. 2006;19:283–97. DOIPubMedGoogle Scholar
- Greub G, Raoult D. Parachlamydiaceae: potential emerging pathogens. Emerg Infect Dis. 2002;8:625–30.PubMedGoogle Scholar
- Dilbeck PM, Evermann JF, Crawford TB, Ward AC, Leathers CW, Holland CJ, Isolation of a previously undescribed rickettsia from an aborted bovine fetus. J Clin Microbiol. 1990;28:814–6.PubMedGoogle Scholar
- Henning K, Schares G, Granzow H, Polster U, Hartmann M, Hotzel H, Neospora caninum and Waddlia chondrophila strain 2032/99 in a septic stillborn calf. Vet Microbiol. 2002;85:285–92. DOIPubMedGoogle Scholar
- Borel N, Thoma R, Spaeni P, Weilenmann R, Teankum K, Brugnera E, Chlamydia-related abortions in cattle from Graubunden, Switzerland. Vet Pathol. 2006;43:702–8. DOIPubMedGoogle Scholar
- Casson N, Entenza JM, Greub G. Serological cross-reactivity between different Chlamydia-like organisms. J Clin Microbiol. 2007;45:234–6. DOIPubMedGoogle Scholar
- Borel N, Mukhopadhyay S, Kaiser C, Sullivan ED, Miller RD, Timms P, Tissue MicroArray (TMA) analysis of normal and persistent Chlamydophila pneumoniae infection. BMC Infect Dis. 2006;6:152. DOIPubMedGoogle Scholar
- Greub G, Raoult D. Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: an electron micrograph study. Appl Environ Microbiol. 2002;68:3076–84. DOIPubMedGoogle Scholar
- Casson N, Medico N, Bille J, Greub G. Parachlamydia acanthamoebae enters and multiplies within pneumocytes and lung fibroblasts. Microbes Infect. 2006;8:1294–300. DOIPubMedGoogle Scholar
- Greub G, Mege JL, Raoult D. Parachlamydia acanthamoebae enters and multiplies within human macrophages and induces their apoptosis. Infect Immun. 2003;71:5979–85. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 13, Number 12—December 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Gilbert Greub, Institute of Microbiology, University of Lausanne, 1011 Lausanne, Switzerland;
Top