Volume 15, Number 5—May 2009
Research
New Respiratory Enterovirus and Recombinant Rhinoviruses among Circulating Picornaviruses
Abstract
Rhinoviruses and enteroviruses are leading causes of respiratory infections. To evaluate genotypic diversity and identify forces shaping picornavirus evolution, we screened persons with respiratory illnesses by using rhinovirus-specific or generic real-time PCR assays. We then sequenced the 5′ untranslated region, capsid protein VP1, and protease precursor 3CD regions of virus-positive samples. Subsequent phylogenetic analysis identified the large genotypic diversity of rhinoviruses circulating in humans. We identified and completed the genome sequence of a new enterovirus genotype associated with respiratory symptoms and acute otitis media, confirming the close relationship between rhinoviruses and enteroviruses and the need to detect both viruses in respiratory specimens. Finally, we identified recombinants among circulating rhinoviruses and mapped their recombination sites, thereby demonstrating that rhinoviruses can recombine in their natural host. This study clarifies the diversity and explains the reasons for evolution of these viruses.
Human rhinoviruses (HRVs) and enteroviruses (HEVs) are leading causes of infection in humans. These 2 picornaviruses share an identical genomic organization, have similar functional RNA secondary structures, and are classified within the same genus (www.ictvonline.org/virusTaxonomy.asp) because of their high sequence homology (1). However, despite their common genomic features, these 2 groups of viruses have different phenotypic characteristics. In vivo, rhinoviruses are restricted to the respiratory tract, whereas enteroviruses infect primarily the gastrointestinal tract and can spread to other sites such as the central nervous system. However, some enteroviruses exhibit specific respiratory tropism and thus have properties similar to rhinoviruses (2–5). In vitro, most HRVs and HEVs differ by their optimal growth temperature, acid tolerance, receptor usage, and cell tropism. The genomic basis for these phenotypic differences between similar viruses is not yet fully understood.
HRVs and HEVs are characterized by ≈100 serotypes. Recently, molecular diagnostic tools have shown that this diversity expands beyond those predefined serotypes and encompasses also previously unrecognized rhinovirus and enterovirus genotypes. As an example, a new HRV lineage named HRV-C was recently identified and now complements the 2 previously known A and B lineages (6–8) (N.J. Knowles, pers. comm.). The C lineage has not only a distinct phylogeny (9–16) but is also characterized by specific cis-acting RNA structures (17).
In this study, we screened a large number of persons with acute respiratory diseases by using assays designed to overcome the diversity of both rhinoviruses and enteroviruses circulating in humans. Whenever possible, we systematically sequenced 5′ untranslated region (UTR), capsid protein VP1, and protease precursor 3CD regions of strains. Our goals were 1) to characterize the diversity of circulating rhinoviruses and, to a lesser extent, enteroviruses, to identify putative new picornavirus variants, and 2) to assess whether recombination may drive HRV evolution, which has not been shown in natural human infections (18).
RNA Extraction, Reverse Transcription-PCR, and Real-Time PCR
Reverse transcription–PCR (Superscript II; Invitrogen, Carlsbad, CA, USA) was performed on RNA extracted by using the HCV Amplicor Specimen Preparation kit (Roche, Indianapolis, IN, USA), TRIzol (Invitrogen), or the QIAamp Viral RNA Mini kit (QIAGEN, Valencia, CA, USA). Real-time PCR specific for HRV-A, HRV-B, and HEV (19), and a generic panenterhino real-time PCR (forward primer 5′-AGCCTGCGTGGCKGCC-3′, reverse primer 5′-GAAACACGGACACCCAAAGTAGT-3′, and probe 5-FAM-CTCCGGCCCCTGAATGYGGCTAA-TAMRA-3′), were performed in several cohort studies (Table).
Clinical Specimens
Picornavirus-positive samples were detected from patients enrolled in cohort studies in different regions of Switzerland during 1999–2008. The main characteristics of these populations, type of respiratory specimens, and screening methods are shown in the Table. The rhinovirus serotypes used for 3CD sequencing were obtained from the American Type Culture Collection (Manassas, VA, USA).
PCR and Sequencing
Sequencing was performed directly from the clinical specimen except for samples selected by routine isolation methods on human embryonic (HE) primary fibroblast cell lines (Table) or for HRV reference serotypes. Primers used to amplify the 5′-UTR and the VP1 and 3CD regions are listed in Technical Appendix 1 Table 1A.
Full-length genome sequences of CL-1231094, a related clinical strain of enterovirus, and partial sequences of CL-Fnp5 and CL-QJ274218 were obtained as follows. RNA extracted by using the QIAamp Viral RNA Mini kit (QIAGEN) plus DNase treatment or with Trizol was reverse transcribed with random-tagged primer FR26RV-N and amplified with the SMART RACE cDNA Amplification kit (Clontech, Mountain View, CA, USA) with a specific forward primer and FR20RV reverse primer (Technical Appendix 1 Table 1B) (23). Amplification products were separated by electrophoresis on agarose gels and fragments (0.6–2.5 kb) were extracted by using the QIAquick Gel Extraction kit (QIAGEN). Purified products were cloned by using the TOPO TA cloning kit (Invitrogen).
Minipreps were prepared from individual colonies and clones with the largest inserts were chosen for sequencing. Sequences obtained were used to design a new forward primer (Technical Appendix 1 Table 1) to advance toward the 3′ end of the genome. PCR products of 3′ genomic ends were obtained by using the BD Smart Race cDNA amplification kit (Becton Dickinson, Franklin Lakes, NJ, USA) according to manufacturer’s instructions. All PCR products were purified by using microcon columns (Millipore, Billerica, MA, USA) and sequenced by using the ABI Prism 3130XL DNA Sequencer (Applied Biosystems, Foster City, CA, USA). Chromatograms were imported for proofreading with the vector NTI Advance 10 program (Invitrogen). Overlapping fragments were assembled with the contigExpress module of the vector NTI Advance 10.
Sequence Analysis, Phylogeny, and Bootscanning of Recombinants
Alignments were constructed by using MUSCLE (24) with a maximum of 64 iterations. (For detailed analyses, see http://cegg.unige.ch/picornavirus.) Multiple FastA was converted into PHYLIP format (for tree building) with the EMBOSS program Seqret (25). Trees were built with PhyML (26) by using the general time reversible model, BIONJ for the initial tree, and optimized tree topology and branch lengths. Trees with <50 species and larger trees used 16 and 8 rate categories, respectively. Transition/transversion ratios, proportions of invariant sites, and shape parameters of the γ distribution were estimated.
To investigate the hypothesis of recombination and map the breakpoints, we adapted the bootscanning method (27) as follows. The alignment was sliced into windows of constant size and fixed overlap and a 100-replicate maximum-likelihood (using HRV-93 as an outgroup) was computed for each window. From each tree, the distance between the candidate recombinant and all other sequences was extracted. This extraction yielded a matrix of distances for each window and for each alignment position. A threshold was defined as the lowest distance plus a fraction (15%) of the difference between the highest and lowest distances. The nearest neighbors of the candidate recombinant were defined as sequences at a distance smaller than this threshold. This distance ensured that the nearest neighbor, as well as any close relative, was always included. Possible recombination breakpoints thus corresponded to changes of nearest neighbors. Serotypes included in this analysis represented serotypes close to CL-013775 and CL-073908 on the basis of 5′-UTR and VP1 phlyogenetic trees (Technical Appendix 2 Figure 1, panels A, B), as well as serotypes close to CL-135587 on the basis of VP1 and 3CD phlyogenetic trees (Technical Appendix 2 Figure 1, panels B, C) and whose full-length sequence was available.
Distance matrices were computed from alignments with the distmat program in EMBOSS (http://bioweb2.pasteur.fr/docs/EMBOSS/embossdata.html) by using the Tamura distance correction. This method uses transition and transversion rates and takes into account the deviation of GC content from the expected value of 50%. Gap and ambiguous positions were ignored. Final values were then converted to similarity matrices by subtracting each value from 100.
Screening of Persons with Respiratory Tract Infections
Persons enrolled in several cohorts of children and adults with respiratory infections (Table) were screened for picornavirus by culture isolation on HE cell lines, real-time PCR specific for HRV-A and HRV-B (19), or by a panenterhino real-time PCR designed to theoretically detect all rhinoviruses and enteroviruses with publicly available sequences. Of 1,592 respiratory samples tested by real-time PCR, 248 were virus positive (Table). The 5′-UTR sequences were obtained for 77 real-time PCR or culture-positive samples and VP1 and 3CD sequences for 48 of these (Table; Technical Appendix 1 Table 2). In parallel, the 3CD sequences were identified for all reference serotypes. The results of this screening are summarized in Technical Appendix 1 Table 2, and all sequences are available from GenBank (accession nos. EU840726–EU840988).
On the basis of these results, respiratory infections caused by HRV-B might be less frequent than those caused by HRV-A, and HRV-A infections are distributed among the whole library of reference serotypes. A specific real-time PCR used to detect enteroviruses in respiratory specimens from some of the cohorts studied indicated that these viruses are rare in children (2.5% vs. 6.3% for HRV) and even rarer or absent in adults (0% vs. 24% for HRV) (28).
Phylogeny and Molecular Epidemiology of 5′-UTR
Our genomic analysis of picornaviruses associated with upper or lower respiratory diseases in adults and children indicates that rhinoviruses circulating in the community are widely diverse. The large number of circulating genotypes supports the view that rhinoviruses do not circulate by waves or outbreaks of a given dominant genotype, which might explain the high frequency of reinfection during short periods. As expected, the observed variability is higher for surface capsid proteins, the targets of most immune pressure, and this region remains the only accurate one for genotyping and defining phylogeny. Technical constraints such as the limited amount of clinical specimens, the use of different screening methods, and the need to sequence an unknown target of extreme variability might have limited the representativeness of our sequence collection. Therefore, our study should not be considered as an exhaustive epidemiologic analysis of rhinoviruses and enteroviruses associated with respiratory diseases.
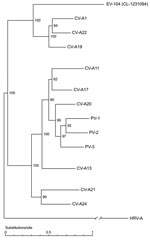
By using a systematic approach, we have identified a new enterovirus genotype (EV-104) that has a divergent 5′-UTR. Undetectable by conventional methods, EV-104 could be detected by using a more generic real-time PCR assay designed to match all known available rhinovirus and enterovirus sequences. Such diagnostic tools have and will lead to constant discovery of new picornavirus genotypes (9–14,16,33–36). These genotypes may represent viruses, in most instances, that have remained undetected because of insensitive cell cultures or overly restrictive molecular tools. In addition, enterovirus genotypes causing respiratory infections, such as EV-68 and CV-A21, might be underrepresented because enteroviruses are usually searched for in fecal specimens (37).
EV-104 belongs to the HEV-C species: CV-A19, CV-A22, and CV-A1 are its closest serotypes. These HEV-C subgroup viruses are genetically distinct from all other serotypes of the species. These viruses show no evidence of recombination with other HEV-C strains and, similar to EV-104, do not grow in cell culture (29). On the basis of our epidemiologic data, we conclude that EV-104 was found in 8 children from different regions of Switzerland who had respiratory illnesses such as acute otitis media or pneumonia. Future studies using adapted detection tools will provide more information on the range of this virus. On the basis of its genomic features and similarities with coxsackieviruses and poliovirus, EV-104 could theoretically infect the central nervous system (2,38). Detection of new subtypes of picornaviruses indicates that viruses with new phenotypic traits could emerge, and conclusions on tropism of new strains should be substantiated by extensive experimental or clinical investigations (39).
By completing the sequence of a seemingly divergent rhinovirus (13), we assigned this virus to the new HRV-C species, thus limiting currently to 3 the number of HRV species. For the sake of simplicity, we propose to consider this virus as a member of the HRV-C clade.
Finally, we demonstrated that rhinovirus evolves by recombination in its natural host. Known to be a driving force of enterovirus evolution, rhinovirus recombination among clinical strains has never been observed. Two clinical isolates of 40 viruses analyzed resulted from recombination events and their breakpoints were identified within the 5′-UTR sequence and the N terminus of protein 3C, respectively. These findings are consistent with the fact that recombination breakpoints in picornaviruses are restricted to nonstructural regions of the genome or between the 5′-UTR and the capsid-encoding region (40). Our observations provide new insight on the diversity and ability of rhinovirus to evolve in its natural host. The fact that only 2 of 40 analyzed viruses over a 9-year period were recombinants is suggestive of a lower recombination frequency in rhinoviruses than in other picornaviruses (32,40) and might be related, but not exclusively, to the short duration of rhinovirus infection (18,31,32). Recombination events occurred between HRV-A genotypes, but whether they can occur in species B and C remains unknown. Interspecies recombination is rare in picornaviruses and is mainly the result of in vitro experiments. For rhinoviruses, the different location of cre elements in each species might be an additional limiting constraint (17).
In summary, we have highlighted the large genomic diversity of the most frequent human respiratory viral infection. Our phylogenetic analysis has characterized circulating strains relative to reference strains and has identified a previously unknown enterovirus genotype. We have shown that recombination also contributes to rhinovirus evolution in its natural environment.
Dr Tapparel is a molecular virologist at the University Hospitals of Geneva. Her research interests are the molecular epidemiology of picornaviruses (rhinoviruses and enteroviruses), development of new diagnostic methods, and determination of fundamental aspects of these viruses.
Acknowledgments
We thank Rosemary Sudan for editorial assistance and the Swiss Institute of Bioinformatics’ Vital-IT facility for bootscanning and computing infrastructure.
This study was supported by the Swiss National Science Foundation (grants 3200B0-101670 to L.K. and 3100A0112588/I to E.Z.), the Department of Medicine of the University Hospitals of Geneva, the University of Geneva Dean’s Program for the Promotion of Women in Science (C.T.), and the Infectigen Foundation.
References
- Tapparel C, Junier T, Gerlach D, Cordey S, Van Belle S, Perrin L, New complete genome sequences of human rhinoviruses shed light on their phylogeny and genomic features. BMC Genomics. 2007;8:224. DOIPubMedGoogle Scholar
- Newcombe NG, Andersson P, Johansson ES, Au GG, Lindberg AM, Barry RD, Cellular receptor interactions of C-cluster human group A coxsackieviruses. J Gen Virol. 2003;84:3041–50. DOIPubMedGoogle Scholar
- Pulli T, Koskimies P, Hyypia T. Molecular comparison of coxsackie A virus serotypes. Virology. 1995;212:30–8. DOIPubMedGoogle Scholar
- Dufresne AT, Gromeier M. A nonpolio enterovirus with respiratory tropism causes poliomyelitis in intercellular adhesion molecule 1 transgenic mice. Proc Natl Acad Sci U S A. 2004;101:13636–41. DOIPubMedGoogle Scholar
- Oberste MS, Maher K, Schnurr D, Flemister MR, Lovchik JC, Peters H, Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol. 2004;85:2577–84. DOIPubMedGoogle Scholar
- Ledford RM, Patel NR, Demenczuk TM, Watanyar A, Herbertz T, Collett MS, VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J Virol. 2004;78:3663–74. DOIPubMedGoogle Scholar
- Laine P, Blomqvist S, Savolainen C, Andries K, Hovi T. Alignment of capsid protein VP1 sequences of all human rhinovirus prototype strains: conserved motifs and functional domains. J Gen Virol. 2006;87:129–38. DOIPubMedGoogle Scholar
- Savolainen C, Blomqvist S, Mulders MN, Hovi T. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J Gen Virol. 2002;83:333–40.PubMedGoogle Scholar
- Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–40. DOIPubMedGoogle Scholar
- Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–25. DOIPubMedGoogle Scholar
- Lamson D, Renwick N, Kapoor V, Liu Z, Palacios G, Ju J, MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194:1398–402. DOIPubMedGoogle Scholar
- Lau SK, Yip CC, Tsoi HW, Lee RA, So LY, Lau YL, Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–64. DOIPubMedGoogle Scholar
- Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2:e966. DOIPubMedGoogle Scholar
- McErlean P, Shackelton LA, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J Clin Virol. 2007;39:67–75. DOIPubMedGoogle Scholar
- McErlean P, Shackelton LA, Andrews E, Webster DR, Lambert SB, Nissen MD, Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C). PLoS One. 2008;3:e1847.
- Renwick N, Schweiger B, Kapoor V, Liu Z, Villari J, Bullmann R, A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J Infect Dis. 2007;196:1754–60. DOIPubMedGoogle Scholar
- Cordey S, Gerlach D, Junier T, Zdobnov EM, Kaiser L, Tapparel C. The cis-acting replication elements define human enterovirus and rhinovirus species. RNA. 2008;14:1568–78. DOIPubMedGoogle Scholar
- Savolainen C, Laine P, Mulders MN, Hovi T. Sequence analysis of human rhinoviruses in the RNA-dependent RNA polymerase coding region reveals large within-species variation. J Gen Virol. 2004;85:2271–7. DOIPubMedGoogle Scholar
- Deffernez C, Wunderli W, Thomas Y, Yerly S, Perrin L, Kaiser L. Amplicon sequencing and improved detection of human rhinovirus in respiratory samples. J Clin Microbiol. 2004;42:3212–8. DOIPubMedGoogle Scholar
- Regamey N, Kaiser L, Roiha HL, Deffernez C, Kuehni CE, Latzin P, Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J. 2008;27:100–5.PubMedGoogle Scholar
- Garbino J, Gerbase MW, Wunderli W, Deffernez C, Thomas Y, Rochat T, Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med. 2004;170:1197–203. DOIPubMedGoogle Scholar
- Kronenberg A, Zucs P, Droz S, Muhlemann K. Distribution and invasiveness of Streptococcus pneumoniae serotypes in Switzerland, a country with low antibiotic selection pressure, from 2001 to 2004. J Clin Microbiol. 2006;44:2032–8. DOIPubMedGoogle Scholar
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102:12891–6. DOIPubMedGoogle Scholar
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7. DOIPubMedGoogle Scholar
- Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–7. DOIPubMedGoogle Scholar
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. DOIPubMedGoogle Scholar
- Salminen MO, Carr JK, Burke DS, McCutchan FE. Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning. AIDS Res Hum Retroviruses. 1995;11:1423–5. DOIPubMedGoogle Scholar
- Garbino J, Soccal PM, Aubert JD, Rochat T, Meylan P, Thomas Y, Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax. 2009; [Epub ahead of print].
- Brown B, Oberste MS, Maher K, Pallansch MA. Complete genomic sequencing shows that polioviruses and members of human enterovirus species C are closely related in the noncapsid coding region. J Virol. 2003;77:8973–84. DOIPubMedGoogle Scholar
- Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–704. DOIPubMedGoogle Scholar
- Kistler AL, Webster DR, Rouskin S, Magrini V, Credle JJ, Schnurr DP, Genome-wide diversity and selective pressure in the human rhinovirus. Virol J. 2007;4:40. DOIPubMedGoogle Scholar
- Simmonds P. Recombination and selection in the evolution of picornaviruses and other mammalian positive-stranded RNA viruses. J Virol. 2006;80:11124–40. DOIPubMedGoogle Scholar
- Smura T, Blomqvist S, Paananen A, Vuorinen T, Sobotova Z, Bubovica V, Enterovirus surveillance reveals proposed new serotypes and provides new insight into enterovirus 5′-untranslated region evolution. J Gen Virol. 2007;88:2520–6. DOIPubMedGoogle Scholar
- Oberste MS, Maher K, Michele SM, Belliot G, Uddin M, Pallansch MA. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species Human enterovirus A. J Gen Virol. 2005;86:445–51. DOIPubMedGoogle Scholar
- Junttila N, Leveque N, Kabue JP, Cartet G, Mushiya F, Muyembe-Tamfum JJ, New enteroviruses, EV-93 and EV-94, associated with acute flaccid paralysis in the Democratic Republic of the Congo. J Med Virol. 2007;79:393–400. DOIPubMedGoogle Scholar
- Norder H, Bjerregaard L, Magnius L, Lina B, Aymard M, Chomel JJ. Sequencing of ‘untypable’ enteroviruses reveals two new types, EV-77 and EV-78, within human enterovirus type B and substitutions in the BC loop of the VP1 protein for known types. J Gen Virol. 2003;84:827–36. DOIPubMedGoogle Scholar
- Witso E, Palacios G, Cinek O, Stene LC, Grinde B, Janowitz D, High prevalence of human enterovirus a infections in natural circulation of human enteroviruses. J Clin Microbiol. 2006;44:4095–100. DOIPubMedGoogle Scholar
- Jiang P, Faase JA, Toyoda H, Paul A, Wimmer E, Gorbalenya AE. Evidence for emergence of diverse polioviruses from C-cluster coxsackie A viruses and implications for global poliovirus eradication. Proc Natl Acad Sci U S A. 2007;104:9457–62. DOIPubMedGoogle Scholar
- Domingo E, Martin V, Perales C, Escarmis C. Coxsackieviruses and quasispecies theory: evolution of enteroviruses. Curr Top Microbiol Immunol. 2008;323:3–32. DOIPubMedGoogle Scholar
- Lukashev AN. Role of recombination in evolution of enteroviruses. Rev Med Virol. 2005;15:157–67. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 15, Number 5—May 2009
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Caroline Tapparel, Laboratory of Virology, Division of Infectious Diseases, University of Geneva Hospitals, 24 Rue Micheli-du-Crest, 1211 Geneva 14, Switzerland
Top