Volume 15, Number 9—September 2009
Dispatch
Human Plasmodium knowlesi Infection Detected by Rapid Diagnostic Tests for Malaria
Abstract
We describe a PCR-confirmed case of Plasmodium knowlesi infection with a high parasitemia level and clinical signs of severe malaria in a migrant worker from Malaysian Borneo in the Netherlands. Investigations showed that commercially available rapid antigen tests for detection of human Plasmodium infections can detect P. knowlesi infections in humans.
The malaria parasite Plasmodium knowlesi naturally occurs in long-tailed and pig-tailed macaques that inhabit forested areas in Southeast Asia. P. knowlesi can be transmitted from monkeys to humans by the bite of an infected mosquito (1), but infection with P. knowlesi was traditionally regarded as a rare disease, occurring only sporadically in humans. However, recent findings of a large number of infected patients in Malaysian Borneo; other reports of human cases in Thailand, Myanmar, the Philippines, and Singapore; and some reports of P. knowlesi malaria acquired by travelers to the Malaysian Borneo suggest that P. knowlesi may be more widespread among humans than previously thought (2–7).
Unfortunately, microscopic analysis of asexual stages of P. knowlesi can misidentify these parasites as P. malariae (3,4). Unlike P. malariae, which multiplies every 3 days in the blood and never results in severe infections, P. knowlesi multiplies daily, and high parasitemia with death in humans can occur (4). Therefore, early diagnosis and immediate treatment is warranted. Although PCR and sequencing are used in species confirmation, a more rapid diagnostic test would be a useful tool for delivering prompt and adequate medical treatment. We report a case of imported P. knowlesi infection in the Netherlands in a migrant worker from Malaysian Borneo. This P. knowlesi infection was detected by commercially available rapid diagnostic antigen tests for malaria.
A 38-year-old man came to the Netherlands in January 2009 to work as a rigger in the harbor of Rotterdam. Since October 2008, he had lived in Kapit, Sarawak, in Borneo and hunted wild animals in the surrounding jungles. One week after arriving in the Netherlands, he came to our hospital with a 5-day history of fever, myalgia, headache, and low back pain. His medical history was uneventful, and he had not experienced any previous malaria attacks.
Physical examination showed a temperature of 40.0°C and remarkable jaundice. Abdominal examination showed no abnormalities. Laboratory investigations showed a moderate anemia (hemoglobin 7.8 mmol/L [reference range 8.5–11.0 mmol/L]), a normal leukocyte count (5.8 × 109/L [reference range 4.3–10 × 109/L]), thrombocytopenia (platelet count 22 × 109/L [reference range 150–400 × 109/L]), an increased level of C-reactive protein (158 mg/L [reference range <10 mg/L]), and liver function abnormalities (serum alanine aminotransferase 199 U/L [reference range <41 U/L]; aspartate aminotransferase 128 U/L [reference range <37 U/L]; lactate dehydrogenase [LDH] 1,059 U/L [reference range <450 U/L]; gamma-glutamyltransferase 183 U/L [reference range <50 U/L]; alkaline phosphatase 285 U/L [reference range <120 U/L]; and total bilirubin 99 μmol/L [reference range <17 μmol/L]). Plasma lactate level was within normal limits.
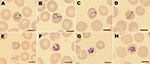
In a rapid diagnostic test for malaria (BinaxNOW Malaria Test; Binax, Inc., Scarborough, ME, USA), his blood sample was negative for P. falciparum histidine-rich protein 2 but showed a positive reaction with pan-malarial aldolase antigen, which suggested a non–P. falciparum infection. Results of quantitative buffy coat analysis were positive for malaria trophozoites, schizonts, and gametocytes. A thin blood film showed parasite density of 2% infected erythrocytes (84,000 trophozoites/μL), schizonts, and gametocytes with an inconclusive morphologic appearance (Figure 1). A P. knowlesi infection was suspected because of his recent stay in Kapit, Malaysian Borneo. The patient was treated orally with chloroquine, 10 mg/kg, followed by 5 mg/kg after 6, 24, and 48 hours, which resulted in a rapid relief of symptoms and fever. Results of quantitative buffy coat analysis, pan-malarial aldolase antigen reactivity, and thick and thin blood smears were negative within 40 hours after administration of chloroquine.
Subsequently, PCR analysis of blood samples taken at admission was performed to determine the Plasmodium species. Human Plasmodium species were excluded by using a conventional nested PCR and real-time PCR (8,9). In addition, PCR analysis was performed on a blood sample by using diagnostic primers for Plasmodium small subunit (SSU) rRNA as described (3), including genus-specific and species-specific primers. In contrast to the method described by Singh et al. (3), nested PCR was not necessary because of high parasitemia and availability of fresh material. Instead, PCRs were performed directly on 2 μL of blood in 25-μL volumes by using the Phusion Blood PCR kit (Finnzymes, Espoo, Finland). Genus-specific primer sets and P. knowlesi–specific primers generated PCR products, providing evidence that the patient had P. knowlesi malaria.
To confirm the PCR result, we sequenced the cloned amplification product generated with primers rPLU1 and rPLU5. Sequences were compared with known Plasmodium A-type SSU rRNA sequences by using the neighbor-joining method (Figure 2). The sequence of the clinical isolate PkHHR-BPRC1 (GenBank accession no. FJ804768) clustered strongly with P. knowlesi A-type SSU RNA sequences, confirming that the patient was infected with the P. knowlesi parasite.
Imported malaria is relatively rare in industrialized countries. Obtaining a correct diagnosis of malaria may be troublesome in centers where laboratory staff are less skilled in the proper identification and quantification of causative Plasmodium species, as may occur in countries in which malaria is not endemic. These centers often use commercially available rapid diagnostic tests to diagnose malaria. In contrast to our case, Bronner et al. reported that the BinaxNOW Malaria test did not detect a P. knowlesi infection in a traveler from Sweden who had a P. knowlesi infection acquired in Malaysian Borneo (2). Low parasitemia (0.1%) in this patient may have caused the lack of reactivity with the pan-malarial antigen aldolase (2).
We evaluated the BinaxNOW Malaria and the OptiMAL Rapid Malaria (Diamed, Cressier, Switzerland) tests for detection of P. knowlesi in human blood by analysis of consecutive blood samples taken after admission. These samples were stored at –20°C for 2 weeks until tests were performed (Table). The blood sample taken on admission (2% infected erythrocytes) did not react with P. falciparum–specific antibody against histidine-rich protein 2, but reacted with the pan-malarial antigen aldolase in the BinaxNOW Malaria Test. This sample also showed a positive result in the P. falciparum–specific LDH and pan-malarial LDH in the OptiMAL Rapid Malaria test, confirming the cross-reactivity of P. knowlesi LDH with monoclonal antibody 17E4 against P. falciparum LDH, as shown by McCutchan et al. (10). This antibody is also used in the OptiMAL Rapid Malaria test (Diamed, pers. comm.). Therefore, a positive test result for the P. falciparum LDH in the OptiMAL Rapid Malaria test is not specific for P. falciparum because it can also be caused by a P. knowlesi infection. The positive result for LDH and aldolase in either test became negative after treatment (Table), which indicates rapid clearance of parasites after treatment. Results of our comparative study suggest that the OptiMAL Rapid Malaria test may be able to detect lower levels of P. knowlesi parasitemia than the BinaxNOW Malaria test.
Our results indicate that commercially available rapid diagnostic antigen tests for human Plasmodium species can detect P. knowlesi infections in humans, although infections with a low parasitemia will not be detected. A negative test result does not exclude a P. knowlesi infection, as it does not exclude infections by other human Plasmodium species (11).
Dr van Hellemond is head of the Laboratory for Parasitology at Erasmus University Medical Centre and Rotterdam Harbour Hospital. His research interests focus on host–parasite interactions, target identification for antiparasitic drugs, and clinical parasitology.
Acknowledgment
We thank Ton van den Berg for preparing blood films and detecting the P. knowlesi infection and Ernst Verschoor for helping with phylogenetic analysis of SSU rRNA sequences.
References
- Chin W, Contacos PG, Collins WE, Jeter MH, Alpert E. Experimental mosquito transmission of Plasmodium knowlesi to man and monkey. Am J Trop Med Hyg. 1968;17:355–8.PubMedGoogle Scholar
- Bronner U, Divis PC, Farnert A, Singh B. Swedish traveller with Plasmodium knowlesi malaria after visiting Malaysian Borneo. Malar J. 2009;8:15. DOIPubMedGoogle Scholar
- Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–24. DOIPubMedGoogle Scholar
- Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratman S, Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–71. DOIPubMedGoogle Scholar
- Kantele A, Marti H, Felger I, Muller D, Jokiranta TS. Monkey malaria in a European traveler returning from Malaysia. Emerg Infect Dis. 2008;14:1434–6. DOIPubMedGoogle Scholar
- Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Pei SW, Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14:814–6. DOIPubMedGoogle Scholar
- Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis. 2004;10:2211–3.PubMedGoogle Scholar
- Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–92. DOIPubMedGoogle Scholar
- Muller-Stover I, Verweij JJ, Hoppenheit B, Gobels K, Haussinger D, Richter J. Plasmodium malariae infection in spite of previous anti-malarial medication. Parasitol Res. 2008;102:547–50. DOIPubMedGoogle Scholar
- McCutchan TF, Piper RC, Makler MT. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg Infect Dis. 2008;14:1750–2.PubMedGoogle Scholar
- van den Broek I, Hill O, Gordillo F, Angarita B, Hamade P, Counihan H, Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. Am J Trop Med Hyg. 2006;75:1209–15.PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 15, Number 9—September 2009
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Perry J.J. van Genderen, Department of Internal Medicine, Harbour Hospital and Institute for Tropical Diseases, Haringvliet 2, 3011 TD Rotterdam, the Netherlands;
Top