Volume 16, Number 6—June 2010
Research
Evolution of Northeastern and Midwestern Borrelia burgdorferi, United States
Figure
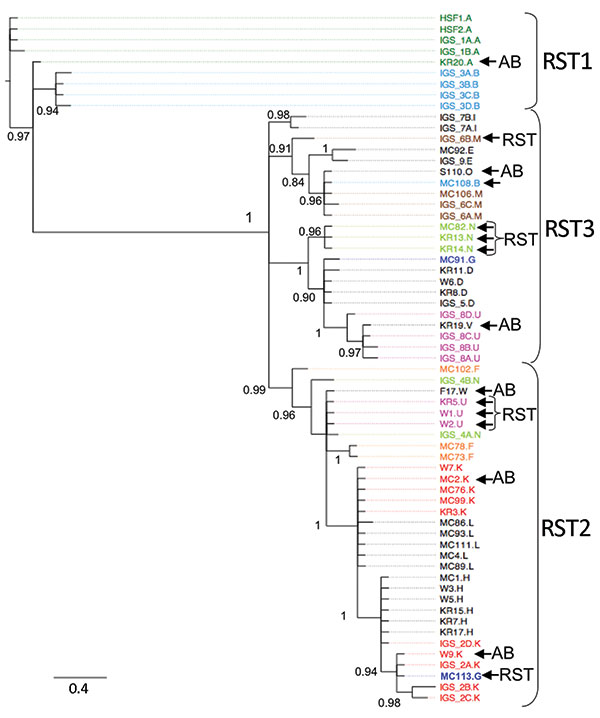
Figure. Phylogeny of Borrelia burgdorferi isolates in the northeastern and midwestern United States based on intergenic spacer (IGS) sequence. operational taxanomic unit names beginning with IGS were isolated in the northeastern United States (10); all other isolates are from patients in the Midwest. The letter after period designates the outer surface protein C (ospC) major allele of the isolate. Colored isolate names highlight isolates with the same ospC major group that cluster in different clades, which suggests horizontal gene transfer. The ospC of several strains is not linked to the IGS ribosomal spacer type (RST) to which it is commonly linked in the Northeast (10,34). AB indicates differences between the ospAB tree and the IGS tree. This tree is midpoint rooted. Scale bar indicates number of substitutions per site.
References
- Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease—United States, 1992–2006. MMWR Surveill Summ. 2008;57:1–9.PubMedGoogle Scholar
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease—a tick-borne spirochetosis. Science. 1982;216:1317–9. DOIPubMedGoogle Scholar
- Gatewood AG, Liebman KA, Vourc’h G, Bunikis J, Hamer SA, Cortinas R, Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl Environ Microbiol. 2009;75:2476–83. DOIPubMedGoogle Scholar
- Brisson D, Dykhuizen DE. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics. 2004;168:713–22. DOIPubMedGoogle Scholar
- Caporale DA, Johnson CM, Millard BJ. Presence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in southern Kettle Moraine State Forest, Wisconsin, and characterization of strain W97F51. J Med Entomol. 2005;42:457–72. DOIPubMedGoogle Scholar
- Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, Tsao J, Kitron U, Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol. 2006;43:166–76. DOIPubMedGoogle Scholar
- Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics. 2002;160:833–49.PubMedGoogle Scholar
- Humphrey PT, Caporale DA, Brisson D. Uncoordinated biogeography of the Lyme disease pathogen, Borrelia burgdorferi, and its tick vector, Ixodes scapularis. Evolution. 2010. In press. DOIPubMedGoogle Scholar
- Qiu WG, Schutzer SE, Bruno JF, Attie O, Xu Y, Dunn JJ, Genetic exchange and plasmid transfers in Borrelia burgdorferi sensu stricto revealed by three-way genome comparisons and multilocus sequence typing. Proc Natl Acad Sci U S A. 2004;101:14150–5. DOIPubMedGoogle Scholar
- Bunikis J, Tsao J, Berglund J, Fish D, Barbour AG. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–55. DOIPubMedGoogle Scholar
- Dykhuizen DE, Polin DS, Dunn JJ, Wilske B, Preac-Mursic V, Dattwyler RJ, Borrelia burgdorferi is clonal: implications for taxonomy and vaccine development. Proc Natl Acad Sci U S A. 1993;90:10163–7. DOIPubMedGoogle Scholar
- Attie O, Bruno JF, Xu Y, Qiu D, Luft BJ, Qiu WG. Co-evolution of the outer surface protein C gene (ospC) and intraspecific lineages of Borrelia burgdorferi sensu stricto in the northeastern United States. Infect Genet Evol. 2007;7:1–12. DOIPubMedGoogle Scholar
- Dykhuizen DE, Baranton G. The implications of a low rate of horizontal transfer in Borrelia. Trends Microbiol. 2001;9:344–50. DOIPubMedGoogle Scholar
- Guttman DS, Wang PW, Wang IN, Bosler EM, Luft BJ, Dykhuizen DE. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by single-strand conformation polymorphism analysis. J Clin Microbiol. 1996;34:652–6.PubMedGoogle Scholar
- Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. DOIPubMedGoogle Scholar
- Stevenson B, Miller JC. Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J Mol Evol. 2003;57:309–24. DOIPubMedGoogle Scholar
- Zhang J-R, Norris SJ. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun. 1998;66:3698–704.PubMedGoogle Scholar
- Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16:1114–6.
- Wormser GP, Liveris D, Nowakowski J, Nadelman RB, Cavaliere LF, McKenna D, Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–5. DOIPubMedGoogle Scholar
- Caporale DA, Kocher TD. Sequence variation in the outer-surface-protein genes of Borrelia burgdorferi. Mol Biol Evol. 1994;11:51–64.PubMedGoogle Scholar
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. DOIPubMedGoogle Scholar
- Rozas J, Rozas R. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics. 1999;15:174–5. DOIPubMedGoogle Scholar
- Jolley KA, Feil EJ, Chan MS, Maiden MC. Sequence type analysis and recombinational tests (START). Bioinformatics. 2001;17:1230–1. DOIPubMedGoogle Scholar
- Sawyer S. Statistical tests for detecting gene conversion. Mol Biol Evol. 1989;6:526–38.PubMedGoogle Scholar
- Smith JM. Analyzing the mosaic structure of genes. J Mol Evol. 1992;34:126–9. DOIPubMedGoogle Scholar
- Smith JM, Smith NH, O’Rourke M, Spratt BG. How clonal are bacteria? Proc Natl Acad Sci U S A. 1993;90:4384–8. DOIPubMedGoogle Scholar
- Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–24.PubMedGoogle Scholar
- Dykhuizen DE, Brisson D, Sandigursky S, Wormser GP, Nowakowski J, Nadelman RB, The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am J Trop Med Hyg. 2008;78:806–10.PubMedGoogle Scholar
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–8. DOIPubMedGoogle Scholar
- Wormser GP, Brisson D, Liveris D, Hanincová K, Sandigursky S, Nowakowski J, Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–64. DOIPubMedGoogle Scholar
- Liveris D, Wormser GP, Nowakowski J, Nadelman R, Bittker S, Cooper D, Molecular typing of Borrelia burgdorferi from Lyme disease patients by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1996;34:1306–9.PubMedGoogle Scholar
- Livey I, Gibbs CP, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–69. DOIPubMedGoogle Scholar
- Ragan MA. Detection of lateral gene transfer among microbial genomes. Curr Opin Genet Dev. 2001;11:620–6. DOIPubMedGoogle Scholar
- Wang G, Ojaimi C, Wu H, Saksenberg V, Iyer R, Liveris D, Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis. 2002;186:782–91. DOIPubMedGoogle Scholar
- Cromley EK, Cartter ML, Mrozinski RD, Ertel SH. Residential setting as a risk factor for Lyme disease in a hyperendemic region. Am J Epidemiol. 1998;147:472–7.PubMedGoogle Scholar
- Brisson D, Dykhuizen DE. A modest model explains the distribution and abundance of Borrelia burgdorferi strains. Am J Trop Med Hyg. 2006;74:615–22.PubMedGoogle Scholar
- Brisson D, Dykhuizen DE, Ostfeld RS. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc Biol Sci. 2008;275:227–35. DOIPubMedGoogle Scholar