Volume 18, Number 10—October 2012
Research
Wild Birds and Urban Ecology of Ticks and Tick-borne Pathogens, Chicago, Illinois, USA, 2005–2010
Abstract
Bird-facilitated introduction of ticks and associated pathogens is postulated to promote invasion of tick-borne zoonotic diseases into urban areas. Results of a longitudinal study conducted in suburban Chicago, Illinois, USA, during 2005–2010 show that 1.6% of 6,180 wild birds captured in mist nets harbored ticks. Tick species in order of abundance were Haemaphysalis leporispalustris, Ixodes dentatus, and I. scapularis, but 2 neotropical tick species of the genus Amblyomma were sampled during the spring migration. I. scapularis ticks were absent at the beginning of the study but constituted the majority of ticks by study end and were found predominantly on birds captured in areas designated as urban green spaces. Of 120 ticks, 5 were infected with Borrelia burgdorferi, spanning 3 ribotypes, but none were infected with Anaplasma phagocytophilum. Results allow inferences about propagule pressure for introduction of tick-borne diseases and emphasize the large sample sizes required to estimate this pressure.
Wild birds can affect zoonotic disease risk to humans, wildlife, and domestic animals through their mobility and influence on the distribution and abundance of pathogens and vectors. Most notably, avian migration allows for rapid transcontinental transportation of novel pathogens and vectors that may seed new disease foci in receptive environments. For example, the spread of highly pathogenic avian influenza into and throughout most countries in Europe most likely occurred through the movement of migratory birds (1). Infected wild birds also contributed to the spread of West Nile virus (WNV) across North America (2). Thus, models of interseasonal connectivity among areas used by migratory birds can be used to forecast disease spread (3).
Over finer spatial scales, the patterns of bird use by blood-feeding vectors affect the prevalence of vector-borne pathogens. Host variation impacts the survival of vectors that feed on birds rather than on other vertebrates (4), and avian species exhibit differential reservoir competency for vector-borne pathogens (5). In combination, these factors influence disease risk; for example, just a few avian species that are heavily fed upon by mosquitoes and highly competent for WNV apparently drive most WNV transmission (6). Furthermore, host association of strains might help maintain pathogen diversity in some vector-borne diseases systems for which birds play critical roles (7).
Urban environments may promote pathogen transmission through increased host contact rates, high rates of pathogen introduction (i.e., propagule pressure), and warmer microclimates that are favorable to pathogens and vectors (8). These effects, in turn, may elevate disease risk to high-density urban human populations. Across gradients of urbanization, the incidence of some zoonotic pathogens has been found to be highest in urban cores (9). Reduced species richness in urban areas may contribute to elevated risk for diseases that are caused by multihost pathogens with generalist vectors (10), although the associations between biodiversity and disease risk are variable (11).
In humans, Lyme disease and anaplasmosis caused by infection with the bacteria Borrelia burgdorferi and Anaplasma phagocytophilum, respectively, are the 2 most common tick-borne diseases in the midwestern and northeastern United States, and both are emerging among human and canine populations (12,13). In eastern North America, both pathogens are maintained in blacklegged tick (Ixodes scapularis)–rodent cycles (14,15). We investigated the role of birds in the urban ecology of tick-borne zoonotic diseases. Our objectives were to 1) ascertain the prevalence of tick parasitism of birds in residential and urban green spaces in southwestern suburban Chicago, Illinois, USA, during a 6-year period; 2) estimate the infection prevalence of Borrelia spp. and A. phagocytophilum in ticks removed from birds; and 3) characterize the diversity of pathogens in ticks removed from birds by using genetic methods.
Bird Capture
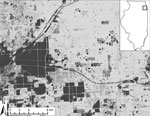
During May–October 2005–2010, birds were captured at 20 field sites in southwestern suburban Chicago (Cook County; 87°44′ W, 41°42′N; Figure). Field sites were categorized as residential sites (n = 14) or urban green spaces (n = 6) and have been described in detail (6). We used 8–10 mist nets (Avinet, Dryden, NY, USA) to capture birds at 7–15 sites per year ≈1 morning per site every 1.5 weeks (2005–2007) or every 3 weeks (2008–2010). For each captured bird, we recorded species, sex, age class (hatch year and after hatch year), and weight, and we attached a numbered leg band before release. All birds were checked for ticks by blowing apart feathers and inspecting the skin, especially around the ears, head, and vent. Ticks were removed and preserved in 70% ethanol. Migratory status of each avian species was assigned (16). Fieldwork was carried out with approvals from animal care review boards at Michigan State University and University of Illinois.
Detection and Typing of Borrelia spp. and A. phagocytophilum
Ticks were identified morphologically to species and stage; a subset was subjected to PCR and sequencing for confirmation (17). All ticks were tested for pathogens, except for 2 specimens that were deposited in the US National Tick Collection (housed at Georgia Southern University, Statesboro, GA, USA) for molecular identification and vouchering. Total DNA from ticks was extracted by using a DNeasy Blood and Tissue Kit (QIAGEN, Valencia, CA, USA) with modifications as described (18). Nymphal ticks were extracted individually, whereas same-species larvae from the same individual animal were pooled. All ticks were tested for the presence of B. burgdorferi sensu stricto and A. phagocytophilum by using a quantitative PCR targeting the 16S rRNA gene (19) and PCR targeting the p44 gene (20), respectively.
B. burgdorferi–positive tick samples were typed by DNA sequencing of both strands of the 16S–23S rRNA intergenic spacer (IGS) region (21); strains were identified, using updated nomenclature (22), to ribosomal spacer type 1, 2, or 3 (23) and IGS subtype by comparing them with the 25 major B. burgdorferi IGS subtypes (21,24). The outer surface protein C (ospC) genotype was inferred on the basis of the linkage disequilibrium between IGS locus and ospC locus (21,22).
Statistical Analyses
Logistic regression was used to assess the variation in tick infestations among years. We used 2- and 3-sample tests for equality of proportions to assess the effects of site category, sex, and age on the prevalence of tick infestations. The Wilson interval with continuity correction was used to estimate the 95% binomial CIs for infection prevalence data. Minimum infection prevalence (i.e., assuming 1 positive larva/pool) was used for tests conducted on pooled larvae. Statistical analyses were performed by using Program R (R Foundation for Statistical Computing, Vienna, Austria).
Bird Captures
We recorded 6,180 total captures, comprising 5,506 individual birds (10.9% recaptures) and 78 species (Table 1). Five species comprised 67% of all captures: Passer domesticus (house sparrow), Turdus migratorius (American robin), Dumetella carolinensis (gray catbird), Spinus tristis (American goldfinch), and Cardinalis cardinalis (northern cardinal). Among all captured birds, 27.3% were known males, 21.3% known females, and 51.3% of unknown sex. The age class was after hatch year for 53.1%, hatch year for 41.8%, and unknown for 5.1% of the birds. Similar numbers of birds were captured from residential sites (3,326, 53.8%) and urban green spaces (2,854, 46.2%). Approximately 2× the number of birds were captured per year in 2005–2007 (1,455 ± 45) as in 2008–2010 (605 ± 159) due to higher mist netting efforts in the initial 3 years of the study.
Tick Prevalence
We removed 357 ticks from 97 individual birds (1 bird with ticks was caught twice), yielding an overall tick infestation prevalence of 1.6% (Table 1). Ticks were usually located beneath the auricular feathers within the skin of the ear canal and second most commonly located in the rictus of the bill and in the skin of the orbital region. Infested birds were collected at 17 of the 20 field sites (11/14 residential sites, 6/6 urban green spaces). Birds with the highest prevalence of infestation (>7% of captures infested) were song sparrows (Melospiza melodia), Swainson’s thrushes (Catharus ustulatus), blue jays (Cyanocitta cristata), ovenbirds (Seiurus aurocapilla), gray-cheeked thrushes (Catharus minimus), and yellow-bellied flycatchers (Empidonax flaviventris) (Table 1).
Most ticks were of 3 species: Haemaphysalis leporispalustris (87.4% of all ticks), Ixodes dentatus (4.8%), and I. scapularis (7.8%). Morphologic and molecular identifications were congruent for all 21 birds subjected to both methods of identification (GenBank accession nos. JQ868565–JQ868585). Overall, 1.3%, 0.1%, and 0.2% of birds were infested with H. leporispalustris, I. dentatus, and I. scapularis, respectively (Table 1). In addition, a single Amblyomma nodosum larva was removed from an after–hatch year Swainson’s thrush on May 17, 2005, and a single A. longirostre nymph was removed from an after–hatch year American redstart (Setophaga ruticilla) on May 18, 2005. The 2 ticks were found on birds captured at site HS (see Figure) during the spring migration. They were identified genetically and vouchered at the US National Tick Collection but not tested for pathogens.
The number of ticks on infested birds ranged from 1 to 23 (median 2 ticks). Of the infested birds, 47% harbored 1 tick and 20% harbored >5 ticks. H. leporispalustris larvae accounted for the greatest tick loads (average 4.3 ticks/bird). Of 98 parasitized birds, 11 (11.2%) were infested with >1 life stage of tick or >1 tick species. Although the overall prevalence of infested birds did not change over the 6-year study (z value = −1.6, df = 6178, p = 0.109), the proportion of infested birds that harbored I. scapularis increased significantly from 0 to 80% (z value = 3.873, df = 96, p = 0.0001), and I. scapularis comprised >90% of ticks removed from birds in the final year of the study. Of the 10 I. scapularis–infested birds, the majority (8) came from urban green spaces (0.28% I. scapularis infestation prevalence across all green spaces), and the minority (2) came from residential sites (0.06% prevalence; z value = 2.2, p = 0.03). Information about the timing of I. scapularis infestation combined with the species and age of the avian host provides evidence for local (Chicago area) acquisition of ticks and for migratory importation of ticks from the north and the south (Table 2).
Tick Infection with B. burgdorferi and A. phagocytophilum
A total of 120 tick samples were tested for pathogens. No ticks tested positive for A. phagocytophilum infection. Five samples tested positive for B. burgdorferi infection: 3 of 6 I. scapularis nymphs (50%, 95% CI 14.0%–86.1%), 1 of 22 I. scapularis larval pools (minimum infection prevalence 4.5%), and 1 of 34 H. leporispalustris nymphs (2.9%, 95% CI 0.2%–17.1%) (Table 3). All 5 positive tick samples were from unique after–hatch year birds of 4 species (American robin, blue jay, red-winged blackbird [Agelaius phoeniceus], Swainson’s thrush) at 4 field sites, including urban green spaces and residential sites. B. burgdorferi 16S–23S rRNA IGS sequences were obtained from all 3 I. scapularis nymphs and represented 3 IGS ribotypes (2, 28, and 14; GenBank accession nos. JQ868562–JQ868564) within ribosomal spacer type 2 and 3; inferred ospC genotypes were H, T, and A3, respectively (Table 3).
The presence of B. burgdorferi–infected I. scapularis ticks on migratory and residential birds in the Chicago region reflects the continued invasion and establishment of this tick and pathogen across the Midwest. In Illinois, as in many other areas of North America (25), there is growing public health concern over the emergence of Lyme disease (26); although, the statewide incidence in Illinois over the study period (1.1 cases/100,000 persons) was an order of magnitude lower than that which characterizes the Lyme disease–endemic regions in the northeastern United States (27). Our study provides evidence of established local populations of I. scapularis ticks in Chicago that may be supplemented by importation of I. scapularis ticks from other populations to the north or south by migratory birds. The Chicago region is a natural corridor for migratory birds, and the risk for tick and pathogen introduction is likely to be elevated on migratory flyways because of seasonal concentrations of birds.
We detected a B. burgdorferi–positive I. scapularis larval pool from a Swainson’s thrush. Given the absence of transovarial transmission in the I. scapularis tick, this finding demonstrates that the Swainson’s thrush can be an infectious reservoir host. On the basis of a limited sample (n = 6), we determined that birds in Chicago harbored B. burgdorferi–infected I. scapularis nymphs at a prevalence (14.0%–86.1%) consistent with that reported for questing nymphs and ticks from birds in Michigan (18), Minnesota (28), and Canada (29). All 3 B. burgdorferi IGS ribotypes present within nymphs in this study have been associated with host-seeking nymphs in Lyme disease–endemic areas of the midwestern and northeastern United States; 2 of the 3 ribotypes were previously detected in larvae removed from birds (30). Two of the ospC types (H and A) presumed present in the collected ticks were among the 4 most invasive genotypes (I, A, H, B) from a study of B. burgdorferi isolates from humans in New York (31). The presence of avian reservoirs and I. scapularis nymphs infected with B. burgdorferi strains capable of causing disseminated human disease supports the possibility that reported cases of human Lyme disease in Chicago residents may result from local exposure to infected I. scapularis ticks. Although none of the ticks removed from birds were positive for A. phagocytophilum, the growing I. scapularis tick population in the region raises the possibility that infection with this pathogen could become an emerging health concern.
Other ticks commonly found on birds in Chicago are I. dentatus and H. leporispalustris ticks, both of which feed almost exclusively on rabbits and birds. I. dentatus ticks are enzootic vectors of B. burgdorferi in regions where I. scapularis ticks do not occur (24). H. leporispalustris ticks transmit Francisella tularensis and spotted-fever group rickettsiae among wildlife (32). In our study, H. leporispalustris ticks had a wide geographic presence across most residential sites and were most commonly found on house sparrows, including 7 hatch-year birds, implying local acquisition in the residential neighborhoods. Neither I. dentatus nor H. leporispalustris ticks regularly infest humans.
We document the presence of 2 neotropical tick species, A. longirostre and A. nodosum, on birds migrating north through Chicago. We note that other species of neotropical Amblyomma ticks have been recovered in the spring on migrant birds in southern Canada (33). A. longirostre and A. nodosum ticks are widely distributed in the neotropical region, and are vectors of Rickettsia amblyommii (34) (which may cause rickettsiosis in humans in North America) (35), R. bellii, and R. parkeri (36). In the United States, R. parkeri is a newly recognized cause of human disease, and a high prevalence of infection (>40% in adults) has been associated with growing populations of Gulf Coast ticks (A. maculatum) (37). Migrant birds from the neotropics likely account for many imports of engorged neotropical ticks and associated pathogens in North America each spring, but a lack of environmental receptivity (host or climatic limitations) has likely prevented establishment.
Data from our large sampling effort show that the dispersal of I. scapularis ticks, B. burgdorferi, and neotropical vector ticks is a rare but detectable event. We sampled several thousand birds and detected I. scapularis ticks on <0.2% and neotropical ticks on <0.05%. However, the rarity of infestations does not mean that infestation is biologically insignificant. Despite the positive relationship between propagule pressure and invasion success, some successful species invasions, especially those of arthropods, can be initiated by a very small number of individuals (38). Low propagule pressure but successful invasion may occur when the environment is receptive to the particular species of ticks and pathogens being dispersed. Indeed, during our study, other researchers showed an increase in the occurrence of B. burgdorferi–infected adult I. scapularis ticks in northwestern Chicago, confirming our prediction (26). Such scenarios of rare introduction but successful establishment of ticks and pathogens pose a major risk for the health of humans, wildlife, and domestic animals in urban environments worldwide.
Sarah A. Hamer is a veterinary ecologist and assistant professor in the Veterinary Integrative Biosciences Department at Texas A&M University. Her research interests include the ecology, evolution, and epidemiology of wildlife and vector-borne and zoonotic diseases.
Acknowledgments
We thank Diane Ghode, Patrick Kelly, Bethany Krebs, and Timothy Thompson for field support; Marilyn Ruiz and Jean Tsao for assisting with project infrastructure and thoughtful discussions; and Lorenza Beati for assisting with tick identification and archiving. We thank the Village of Oak Lawn, Illinois, for providing field laboratory facilities; other Illinois municipalities (Evergreen Park, Palos Hills, Burbank, Alsip, Indian Head Park) and the City of Chicago; and private homeowners for allowing us to conduct this research. We also thank the 2 anonymous peer reviewers whose comments helped to improve our manuscript.
This project was funded through National Science Foundation Ecology of Infectious Disease Grants 0429124 and 0840403.
References
- Kilpatrick AM, Chmura AA, Gibbons DW, Fleischer RC, Marra PP, Daszak P. Predicting the global spread of H5N1 avian influenza. Proc Natl Acad Sci U S A. 2006;103:19368–73. DOIPubMedGoogle Scholar
- Rappole JH, Hubálek Z. Migratory birds and West Nile virus. J Appl Microbiol. 2003;94(Suppl):47S–58S. DOIPubMedGoogle Scholar
- Peterson AT, Andersen MJ, Bodbyl-Roels S, Hosner P, Nyari A, Oliveros C, A prototype forecasting system for bird-borne disease spread in North America based on migratory bird movements. Epidemics. 2009;1:240–9. DOIPubMedGoogle Scholar
- Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, Hosts as ecological traps for the vector of Lyme disease. Proc Biol Sci. 2009;276:3911–9. DOIPubMedGoogle Scholar
- Ginsberg HS, Buckley PA, Balmforth MG, Zhioua E, Mitra S, Buckley FG. Reservoir competence of native North American birds for the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 2005;42:445–9. DOIPubMedGoogle Scholar
- Hamer GL, Chaves LF, Anderson TK, Kitron UD, Brawn JD, Ruiz MO, Fine-scale variation in vector host use and force of infection drive localized patterns of West Nile virus transmission. PLoS ONE. 2011;6:e23767. DOIPubMedGoogle Scholar
- Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 2002;10:74–9. DOIPubMedGoogle Scholar
- Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol. 2007;22:95–102. DOIPubMedGoogle Scholar
- Hamer SA, Lehrer E, Magle SB. Wild birds as sentinels for multiple zoonotic pathogens along an urban to rural gradient in greater Chicago, Illinois. Zoonoses Public Health. 2012;59:355–64. DOIPubMedGoogle Scholar
- Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–52. DOIPubMedGoogle Scholar
- Randolph SE, Dobson AD. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139:847–63. DOIPubMedGoogle Scholar
- Chomel B. Tick-borne infections in dogs—an emerging infectious threat. Vet Parasitol. 2011;179:294–301. DOIPubMedGoogle Scholar
- Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–34. DOIPubMedGoogle Scholar
- Telford SR, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93:6209–14. DOIPubMedGoogle Scholar
- Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–6. DOIPubMedGoogle Scholar
- Walk JW, Ward MP, Benson TJ, Deppe JL, Lischka SA, Bailey SD, Illinois birds: a century of change. Champaign (IL): Illinois Natural History Survey; 2010.
- Poucher KL, Hutcheson HJ, Keirans JE, Durden LA, Black WC. Molecular genetic key for the identification of 17 Ixodes species of the United States (Acari: Ixodidae): a methods model. J Parasitol. 1999;85:623–9. DOIPubMedGoogle Scholar
- Hamer SA, Tsao JI, Walker ED, Hickling GJ. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. EcoHealth. 2010;7:47–63. DOIPubMedGoogle Scholar
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101:18159–64. DOIPubMedGoogle Scholar
- Holden K, Boothby JT, Anand S, Massung RF. Detection of Borrelia burgdorferi, Ehrlichia chaffeensis, and Anaplasma phagocytophilum in ticks (Acari: Ixodidae) from a coastal region of California. J Med Entomol. 2003;40:534–9. DOIPubMedGoogle Scholar
- Bunikis J, Garpmo U, Tsao J, Berglund J, Fish D, Barbour AG. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–55. DOIPubMedGoogle Scholar
- Travinsky B, Bunikis J, Barbour AG. Geographic differences in genetic locus linkages for Borrelia burgdorferi. Emerg Infect Dis. 2010;16:1147–50. DOIPubMedGoogle Scholar
- Liveris D, Gazumyan A, Schwartz I. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1995;33:589–95.PubMedGoogle Scholar
- Hamer SA, Hickling GJ, Sidge JL, Rosen ME, Walker ED, Tsao JI. Diverse Borrelia burgdorferi strains in a bird tick cryptic cycle. Appl Environ Microbiol. 2011;77:1999–2007. DOIPubMedGoogle Scholar
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg. 2012;86:320–7. DOIPubMedGoogle Scholar
- Jobe DA, Nelson JA, Adam MD, Martin SA. Lyme disease in urban areas, Chicago. Emerg Infect Dis. 2007;13:1799–800. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Summary of notifiable diseases. MMWR Morb Mortal Wkly Rep. 2011;58:1–100.PubMedGoogle Scholar
- Weisbrod AR, Johnson RC. Lyme disease and migrating birds in the Saint Croix River Valley. Appl Environ Microbiol. 1989;55:1921–4.PubMedGoogle Scholar
- Ogden NH, Lindsay LR, Hanincova K, Barker IK, Bigras-Poulin M, Charron DF, Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl Environ Microbiol. 2008;74:1780–90. DOIPubMedGoogle Scholar
- Brinkerhoff RJ, Bent SJ, Folsom-O'Keefe CM, Tsao K, Hoen AG, Barbour AG, Genotypic diversity of Borrelia burgdorferi strains detected in Ixodes scapularis larvae collected from North American songbirds. Appl Environ Microbiol. 2010;76:8265–8. DOIPubMedGoogle Scholar
- Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–64. DOIPubMedGoogle Scholar
- Sonenshine DE. Ticks of Virginia. Blacksburg (VA): Virginia Polytechnic Institute and State University, College of Agriculture and Life Sciences; 1979.
- Scott JD, Lee M-K, Fernando K, Durden LA, Jorgensen DR, Mak S, Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J Vector Ecol. 2010;35:124–39. DOIPubMedGoogle Scholar
- Ogrzewalska M, Uezu A, Jenkins CN, Labruna MB. Effect of forest fragmentation on tick infestations of birds and tick infection rates by Rickettsia in the Atlantic forest of Brazil. EcoHealth. 2011;8:320–31. DOIPubMedGoogle Scholar
- Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. DOIPubMedGoogle Scholar
- Ogrzewalska M, Pacheco RC, Uezu A, Richtzenhain LJ, Ferreira F, Labruna MB. Rickettsial infection in Amblyomma nodosum ticks (Acari: Ixodidae) from Brazil. Ann Trop Med Parasitol. 2009;103:413–25. DOIPubMedGoogle Scholar
- Fornadel CM, Zhang X, Smith JD, Paddock CD, Arias JR, Norris DE. High rates of Rickettsia parkeri infection in Gulf Coast ticks (Amblyomma maculatum) and identification of “Candidatus Rickettsia andeanae” from Fairfax County, Virginia. Vector Borne Zoonotic Dis. 2011;11:1535–9. DOIPubMedGoogle Scholar
- Simberloff D. The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst. 2009;40:81–102. DOIGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 18, Number 10—October 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sarah A. Hamer, Veterinary Integrative Bioscience Department, 107-A VMA Building, Texas A&M University, College Station, TX 77843-4458, USA
Top