Volume 18, Number 11—November 2012
CME ACTIVITY - Research
Invasive Pneumococcal Disease and 7-Valent Pneumococcal Conjugate Vaccine, the Netherlands
Figure
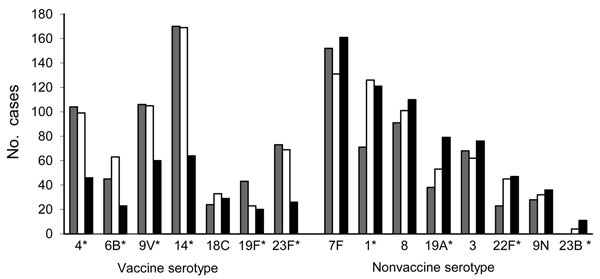
Figure. . . . . . Serotype distribution of invasive pneumococcal disease in the Netherlands before and after (early and late) introduction of the 7-valent pneumococcal conjugate vaccine (PCV7). The 7 vaccine serotypes and the most prevalent nonvaccine serotypes are shown. The cases represent case-patients included in the study (covering ≈25% of the Dutch population). Gray, pre-implementation period (June 2004–May 2006); white, early post-implementation period (June 2006–May 2008); black, late post-implementation period (June 2008–May 2010); *Significant difference (p<0.05) between pre- and post-implementation periods, calculated by the incidence rate ratio.
1These authors contributed equally to this article.
2These authors contributed equally to this article.
3Additional members of the Invasive Pneumococcal Disease Sentinel Surveillance Laboratory Group are listed at the end of this article.
Members of the Invasive Pneumococcal Disease Sentinel Surveillance Laboratory Group: Karola Waar, Izore, Centre for Infectious Diseases Friesland, Leeuwarden, the Netherlands; Bert Mulder, Laboratory of Medical Microbiology Twente Achterhoek, Enschede, the Netherlands; Caroline Swanink, Department of Medical Microbiology and Medical Immunology Hospital Rijnstate, Arnhem, the Netherlands; Bram Diederen, Regional Laboratory of Public Health, Haarlem, the Netherlands; Niek Arents, Laboratory for Pathology and Medical Microbiology, Veldhoven, the Netherlands; Ine Frénay, Regional Laboratory for Medical Microbiology and Infectious Diseases, Dordrecht–Gorinchem, the Netherlands; Hans Wagenvoort, Atrium Medical Center, Heerlen, the Netherlands; Bartelt de Jongh, St. Antonius Hospital, Nieuwegein, the Netherlands; Lodewijk Spanjaard, Academic Medical Center, Amsterdam, the Netherlands