Volume 18, Number 12—December 2012
Research
Reservoir Competence of Wildlife Host Species for Babesia microti
Abstract
Human babesiosis is an increasing health concern in the northeastern United States, where the causal agent, Babesia microti, is spread through the bite of infected Ixodes scapularis ticks. We sampled 10 mammal and 4 bird species within a vertebrate host community in southeastern New York to quantify reservoir competence (mean percentage of ticks infected by an individual host) using real-time PCR. We found reservoir competence levels >17% in white-footed mice (Peromyscus leucopus), raccoons (Procyon lotor), short-tailed shrews (Blarina brevicauda), and eastern chipmunks (Tamias striatus), and <6% but >0% in all other species, including all 4 bird species. Data on the relative contributions of multiple host species to tick infection with B. microti and level of genetic differentiation between B. microti strains transmitted by different hosts will help advance understanding of the spread of human babesiosis.
Human babesiosis is a growing public health concern, especially in the northeastern United States. Babesiosis is a zoonotic, malaria-like illness that can be particularly severe and sometimes fatal in elderly, asplenic, or immunocomprised persons (1). In the lower Hudson Valley region of New York State, 5 locally acquired cases of babesiosis were documented in 2001 (2), and incidence has increased 20-fold from 2001 through 2008 (3).
The causal agent of human babesiosis, Babesia microti (Apicomplexa: Piroplasmida), is a protozoan blood parasite that is transmitted in nature by the bite of an infected tick. In the northeastern United States, the vector of this disease is Ixodes scapularis, the black-legged tick, which is also the primary vector of Borrelia burgdorferi and Anaplasma phagocytophilum, the causal agents of Lyme disease and human granulocytic anaplasmosis, respectively. B. microti is not known to be transmitted transovarially on the basis of available evidence (4–7), indicating that it is not passed from infected adult ticks to eggs, and therefore must be acquired from a blood meal on an infected host. Larval ticks bite infected animals and obtain the pathogen, molt into nymphs, and overwinter. The following year, they then can infect additional hosts when seeking a second blood meal as nymphs or a third blood meal as adults. B. microti has been detected in questing adult and nymphal ticks in studies across the northeastern United States (8–12). In the case of Lyme disease, which is transmitted by the same vector, the nymphal stage is most relevant to human health because bites from nymphs often go undetected and provide greater opportunities to transmit the pathogen (13,14). Babesiosis can also be acquired through blood transfusions, another growing concern for public health (15).
B. microti can infect a range of animal species, but the reservoir competence of many wildlife hosts in the northeastern United States is not well known. We define reservoir competence as the mean percentage of ticks infected by any individual host of a given species. Furthermore, many studies test for the presence of or exposure to the pathogen in host blood or tissue, which provides useful information about host infection, but not information on how often infected animals transmit the pathogen to tick vectors. White-footed mice (Peromyscus leucopus) and meadow voles (Microtus pennsylvanicus) have been established as reservoir hosts on the basis of seroprevalence (16,17), and P. leucopus mice are known to transmit the pathogen to ticks (18). Other rodent species, including species in other genera of mice (Apodemus and Sicista) and voles (Eothenomys, Lagurus, and Myodes) are known hosts in Europe (19–21) and Asia (22–24). B. microti or B. microti–like infection has been observed in other common eastern US mammal species, such as short-tailed shrews (Blarina brevicauda) (25), eastern cottontail rabbits (Sylvilagus floridanus) (17), eastern chipmunks (Tamias striatus) (17), raccoons (Procyon lotor) (26) and foxes (27), and congeneric species in other regions or countries, including Sciurus spp. squirrels (28) and Sorex spp. shrews (29). Birds have not been extensively tested for B. microti, but in Europe, evidence of B. microti infection has recently been discovered in engorged larval ticks that had been feeding on birds of several species (30–32). Determining the role that any of these species play in B. microti dynamics in nature requires information on the rate at which hosts transmit B. microti to tick vectors.
Few systematic surveys have been conducted that compare reservoir competencies of multiple potential host species for B. microti. Testing a broad sample of wildlife species would enable us not only to identify which species transmit the pathogen, but also which species act as weakly competent or incompetent hosts, providing blood meals to ticks but rarely or never transmitting the pathogen (33). Infection of nymphs by other tickborne pathogens, such as B. burgdorferi, is affected by the presence of strongly and weakly competent reservoir hosts in the same communities (34,35). On Nantucket Island, an area well studied for B. microti, multiple hosts transmit the pathogen but differ in infection prevalence (17). Although several studies have extensively sampled small mammal communities for seroprevalence of B. microti (19–23), comprehensive surveys of more diverse host communities, for either seroprevalence or reservoir competence, are rare.
In this study, we sought to determine the level of reservoir competence for B. microti for as many host species as possible in a babesiosis-endemic region (Dutchess County, New York) where human babesiosis cases are rapidly increasing (3). We designed and tested a real-time PCR method to determine whether ticks were infected with B. microti. To determine the relative levels of reservoir competence in as many potential host species as possible, we applied this method to sample newly molted nymphal ticks that fed as larvae on a range of potential wildlife hosts. We tested the hypothesis that white-footed mice (P. leucopus) were the predominant host of B. microti in this community. We also compared these results to levels of prevalence in questing nymphal ticks from the same region. Our overall goal was to improve understanding of the role of multiple wildlife host species in B. microti transmission.
Field Methods
Hosts were trapped on the property of the Cary Institute of Ecosystem Studies (Millbrook, NY, USA) during the peak abundance of larval black-legged ticks (I. scapularis) in July–September in 2008, 2009, and 2010. Hosts included 10 mammal and 4 bird species (Table 1). We focused our sampling efforts on common forest-dwelling terrestrial mammals and ground-dwelling songbirds known to host I. scapularis ticks. Hosts were held for 3 days in cages with wire mesh floors suspended over pans lined with wet paper towels so that ticks could feed to repletion, drop from hosts, and be collected. Our ideal was to sample 10–25 ticks/individual host, but our ability to meet this depended on host tick loads. If hosts did not drop enough ticks within 3 days, we increased sample size when possible for selected individuals by infesting them with unfed larval ticks according to the methods of Keesing et al. (35).
Larval ticks were either collected in the field or hatched from eggs in the laboratory. Larvae hatched from eggs in the laboratory were the offspring of locally collected adult ticks fed on uninfected rabbits. Because transovarial transmission of B. microti is not known to occur (4–7), these infestations did not affect host exposure to the pathogen. Hosts that had been infested were held for an additional 4 days and engorged ticks were collected each day. All engorged larval ticks were held in moistened glass vials at constant temperature and humidity until they molted into the nymphal stage. Newly molted nymphs were flash-frozen in liquid nitrogen and stored at −80°C. All procedures were conducted with approval from the Cary Institute of Ecosystem Studies Institutional Animal Care and Use Committee.
To provide a context for assessing the reservoir competence of hosts at the study site, we also sampled questing nymphal ticks in June 2010 (13 sites) and June 2011 (5 sites) in the towns of Washington, New York, and adjacent Pleasant Valley, New York. We collected questing nymphal ticks and estimated nymphal density by drag sampling (36). Corduroy cloths (1 m2) were dragged along 400-m transects in each site once or twice in a given year during the annual peak in nymphal questing activity. Ticks were counted and collected every 15–30 min. Questing nymphs were flash frozen upon collection and stored as described above. All sites sampled for questing nymphs were in oak-dominated eastern deciduous forests as described by Ostfeld et al. (36) either on the grounds of or within 12 km of the Cary Institute of Ecosystem Studies, where trapping occurred. For each site, the total density of nymphs was multiplied by the proportion of infected nymphs to provide an estimate of the density of infected nymphs.
DNA Extraction and Amplification
Only ticks from hosts that produced a minimum of 10 newly molted nymphs were tested for infection, with the exception of flying squirrels (Glaucomys volans), masked shrews (Sorex cinereus), and American robins (Turdus migratorius), which had low tick loads. For these species, we tested ticks from hosts with >4 newly molted nymphs, but considered these data provisional given low sample sizes per individual host.
To obtain DNA from the ticks, we extracted total genomic DNA using either the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany) or the Gentra Puregene Tissue Kit (QIAGEN). Each DNA extraction included a negative control of unfed larval ticks. We designed 2 primers to amplify a 133-bp fragment of the 18S rDNA region in the B. microti species complex (including all clades within B. microti) (37), smbaJF (5′-GCG TTC ATA AAA CGC AAG GAA GTG T-3′), and smbaKR (5′-TGT AAG ATT ACC CGG ACC CGA CG-3′).
We then amplified DNA in a real-time PCR by using SYBR green technology in a C1000 Thermal Cycler with CFX96 Optical Reaction Module (Bio-Rad, Hercules, CA, USA). The reaction mixture included 12.5 μL iQ SYBR Green Supermix (Bio-Rad), 1.25 μL of 10 μmol/L solutions of each primer, 7.5 μL autoclaved or filter-sterilized ultrapure water, and 2.5 μL of template (undiluted tick extracts). Reaction conditions were 10 min at 95°C, followed by 40 cycles for 10 s at 95°C, 20 s at 68°C, and 40 s at 72°C. As a positive control, we used B. microti isolates from human patient provided by the New York State Department of Health (Albany, NY, USA). DNA extractions from unfed larval ticks and ultrapure water were used as negative controls to account for potential contamination during the extraction and PCR. To prevent contamination between samples, barrier pipette tips were used throughout the process. Three replicate PCRs were run per tick.
We incorporated melting curve analysis after amplification to distinguish true-positive samples from false-positive samples or mispriming. PCR products were heated from 72°C to 90°C; temperature was increased by 0.5°C every 30 s. Positive controls consistently had melting point maxima of 84°C or 84.5°C. To confirm PCR results, a subset of 197 real-time PCR products were sequenced, including samples with melting point maxima close to the range of standards (83.5°C–84.5°C) and representative samples of products amplifying at different melting point maxima from different host species (Table 2). PCR products were purified by using a QIAquick PCR Purification Kit (QIAGEN) and sequenced by using an ABI 3730XL Autosequencer (Applied Biosystems, Carlsbad, CA, USA). Sequences were edited manually by using FinchTV (Geospiza, Seattle, WA, USA), and identity of sequences was confirmed by using basic local alignment search tool (BLAST) searches (National Center for Biotechnology Information, Bethesda, MD, USA) of GenBank and the blastn algorithm (38). Identical molecular protocols were used for analysis of questing nymphs.
Ticks were considered positive for B. microti if any 1 of 3 replicate samples amplified and had a melting point maximum of 83.5°C–84.5°C. Ticks with marginal results (positive replicates with double peaks, positive replicates with a melting point maximum of 83°C, or replicates that amplified and had a melting point maximum of 83.5°C–84.5°C but low fluorescence) were run a second time. If results of the second run met the criteria for positivity described above, ticks were considered positive for B. microti. If results of the second run were marginal or negative, ticks were considered negative for B. microti. Reservoir competence for each host species was calculated as the average percentage of ticks infected per individual host. Hosts were considered infected if they produced >1 infected tick.
We sampled 3,892 ticks from 214 individual hosts for B. microti by real-time PCR (Table 1). We used melting curve analysis and DNA sequencing to confirm efficacy of the real-time PCR. False-positive and false-negative results were rare (Table 2). Of the 79 replicates sequenced with melting point maxima ranging from 83.5°C to 84.5°C, 76 (96.2%) were confirmed as B. microti by sequencing. Of the 118 samples sequenced with melting point maxima outside that range, only 2 (1.7%) samples were confirmed as B. microti by sequencing. Among samples positive for B. microti, 38 of 38 sequences from ticks fed on raccoons (collected from 7 hosts), 3 of 4 sequences from ticks fed on opossums (3 hosts), and 1 sequence from a tick fed on a wood thrush had 2 single bp differences from all other B. microti–positive samples sequenced (1 substitution and 1 insertion) (Table 3). One sequence from a tick fed on a raccoon had 1 additional substitution; this sample had a melting point maximum of 82.5°C.
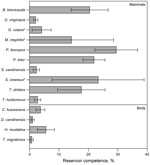
We assessed levels of reservoir competence in 14 host species (10 mammals and 4 birds) (Table 1). White-footed mice, raccoons, short-tailed shrews, and chipmunks had mean levels of reservoir competence >17% (Figure). All other hosts with >10 individuals tested, including opossums, gray and red squirrels, and all 4 species of birds tested (veery [Catharus fuscescens], gray catbird [Dumetella carolinensis], wood thrush [Hylocichia mustelina], and American robin), had mean levels of reservoir competence <6%. Variance in reservoir competence differed significantly among these 11 species (χ2 = 73.6973, df = 10, p = 8.525 × 10−12, by Fligner-Killeen test of homogeneity of variances). Flying squirrels, striped skunks, and masked shrews all transmitted B. microti to ticks, but sample sizes were smaller than those of the other 11 species, leaving us unable to make firm conclusions about relative levels of reservoir competence. There were no host species that did not transmit B. microti to any larval ticks (Figure, Table 4). The percentage of hosts infected ranged from 85.7% (raccoons) to 11.8% (robins), and the average percentage of ticks infected by infected hosts ranged from 4.5% (gray catbirds) to 41.8% (white-footed mice), not including host species with a sample size of <10 individuals (Table 4).
In addition, we tested 414 questing nymphs from 18 field sites for B. microti (mean 23 ticks/site, range 12–34 ticks/site). Mean (SD) prevalence of B. microti was 16.8% (12.2%). Variation in infection prevalence among sites was high, ranging from 0% to 41.4%. Mean (SD) nymphal density among all sites was 6.49 questing nymphs/100 m2 (5.13 questing nymphs/100 m2) and ranged from 0.67 to 16.5 nymphs/100 m2. On the basis of these data, the mean (SD) density of infected nymphs at these 18 sites was 1.21 nymphs/100 m2 (1.62 nymphs/100 m2, range 0–6.83 infected nymphs/100 m2).
Our broad survey of 14 commonly parasitized, co-occurring mammals and birds in a babesiosis-endemic zone showed variation in B. microti reservoir competence among host species. As expected, white-footed mice (P. leucopus) act as a competent reservoir for B. microti, infecting an average of 29.5% of larval ticks. However, other small mammals (B. brevicauda shrews and T. striatus chipmunks) and raccoons (P. lotor) are also relatively competent reservoirs (Figure), showing mean reservoir competence ranging from 17.6% to 21.9%. All other wildlife species with >10 individuals tested had low levels of B. microti transmission; no individual host species infected >6% of ticks on average. Depending on their tick loads and relative abundances, these species have the potential to lower rates of disease risk (i.e., infection prevalence) by feeding ticks but not transmitting this pathogen (35). Within a host species, individual differences such as age, reproductive status, sex, time since infection, and degree of co-infection may affect transmission of B. microti. Our approach focused on sampling a breadth of species under a range of natural conditions rather than sampling any single species in sufficient depth to take potential causes of intraspecific variation into account. Future studies examining the mechanisms controlling individual variation in reservoir capacity are warranted because we observed considerable variation in reservoir capacity between individual species (Table 4).
The average level of B. microti infection in questing nymphal ticks was intermediate between more and less competent reservoir species, consistent with the fact that larval ticks are feeding on a combination of hosts with varying levels of reservoir competence. Variation in B. microti prevalence in questing ticks among sites could reflect differences in host community composition or differences in infection prevalence among hosts at different sites. Site-specific differences in the density of questing nymphs at each site also contribute to variation in the density of infected nymphs among sites. Nymphal densities measured fall within the range documented in previous studies (36). Future studies should focus on sampling questing nymphs more broadly across landscapes to link levels of infection in questing ticks and the density of infected nymphs to host communities and other landscape-scale habitat variation. Monitoring of babesiosis-endemic areas for temporal changes in nymphal infection prevalence may also help in understanding mechanisms for the regional increase in human babesiosis cases.
Ground-dwelling bird species have not been sampled frequently for their capacity to transmit B. microti to ticks, but they might play a role in disease transmission and spread because they are exposed to I. scapularis ticks. Of the 4 species tested in this study, wood thrushes (H. mustelina) had the highest reservoir competence of all birds tested (5.6%), but the other 3 bird species were also able to transmit the pathogen, albeit at low levels (<4%). Transmission of B. microti has been detected in other bird species; positive I. ricinus tick larvae have been found feeding on European robins (Erithacus rubecula) (32) and other birds (30,31).
The real-time PCR developed for this study was effective in amplifying B. microti DNA and had a high level of specificity for this pathogen. The measured rates of false-positive and false-negative results were <5% (Table 2). The low false-positive rate is dependent on melting curve analysis after amplification. False-negative results tended to occur at melting point maxima just below what we considered diagnostic for B. microti (82.5°C–83°C), and may represent strain variation within B. microti. In future studies, it may be necessary to directly sequence samples with these melting point maxima.
On the basis of the short fragments that we sequenced, our samples of B. microti suggest some degree of host specialization (Table 3), consistent with previous work showing divergent clades of B. microti (37,39,40). Variation found in raccoon and opossum isolates matches variation in other raccoon isolates (GenBank accession nos. AY144701 and AB197940). We stress the preliminary nature of this result given the short sequence length and the relative paucity of B. microti sequences available in GenBank. The role of raccoons and opossums in human babesiosis dynamics depends on the zoonotic potential of the B. microti strains they carry. Phylogenetic analyses of 18S and β-tubulin genes (37) and the chaperonin-containing t-complex polypeptide l gene (39) of B. microti showed a distinction between raccoon isolates and known zoonotic strains. Given that all host species sampled transmitted B. microti, further studies detailing the level of genetic differentiation between B. microti samples isolated from different hosts are critical, as is a thorough assessment of the genetic diversity of B. microti infections of humans. Improved understanding of B. microti strain diversity is necessary to consider the public health implications of the role of different hosts in B. microti dynamics.
In this study, we demonstrated that P. leucopus mice are a competent reservoir host for B. microti, but other small mammals and raccoons have comparable reservoir competence and might play a critical role in disease transmission, depending on their tick loads and relative abundance (34,35). At least 1 individual of all wildlife host species sampled transmitted B. microti to >1 I. scapularis tick, even when host sample sizes were relatively low. Tick vectors that transmit this pathogen interact with strongly and weakly competent reservoir hosts, and this variation in reservoir competence within host communities should be considered when predicting risk for infection with B. microti based on animal community composition. To explain the recent emergence of human babesiosis, the community ecology of B. microti needs to be understood in greater depth.
Dr Hersh is a postdoctoral researcher at Bard College, Annandale-on-Hudson, NY, and the Cary Institute of Ecosystem Studies, Millbrook, NY. Her research interest is linkages between biodiversity and disease.
Acknowledgments
We thank Shannon Duerr, Diana McHenry, Laura Cheney, Mitch Le Sage, Kelly Oggenfuss, Chris Neill, and all 2008–2011 research assistants in the Ostfeld Laboratory for laboratory and field assistance; and Jorge Durán Humia and M. Andrea Previtali for useful comments on the manuscript.
This study was supported by National Science Foundation grants 0940830 and 0813041.
References
- Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–69. DOIPubMedGoogle Scholar
- Kogut SJ, Thill CD, Prusinski MA, Lee JH, Backenson PB, Coleman JL, Babesia microti, upstate New York. Emerg Infect Dis. 2005;11:476–8. DOIPubMedGoogle Scholar
- Joseph JT, Roy SS, Shams N, Visintainer P, Nadelman RB, Hosur S, Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843–7. DOIPubMedGoogle Scholar
- Gray J, Zintl A, Hildebrandt A, Hunfeld K-P, Weiss L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1:3–10. DOIPubMedGoogle Scholar
- Gray J, von Stedingk LV, Gürtelschmid M, Granström M. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J Clin Microbiol. 2002;40:1259–63. DOIPubMedGoogle Scholar
- Oliveira MR, Kreier JP. Transmission of Babesia microti using various species of ticks as vectors. J Parasitol. 1979;65:816–7. DOIPubMedGoogle Scholar
- Tokarz R, Jain K, Bennett A, Briese T, Lipkin WI. Assessment of polymicrobial infections in ticks in New York State. Vector Borne Zoonotic Dis. 2010;10:217–21. DOIPubMedGoogle Scholar
- Adelson ME, Rao R-VS, Tilton RC, Cabets K, Eskow E, Fein L, Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in northern New Jersey. J Clin Microbiol. 2004;42:2799–801. DOIPubMedGoogle Scholar
- Holman MS, Caporale DA, Goldberg J, Lacombe E, Lubelczyk C, Rand PW, Anaplasma phagocytophilum, Babesia microti, and Borrelia burgdorferi in Ixodes scapularis, southern coastal Maine. Emerg Infect Dis. 2004;10:744–6. DOIPubMedGoogle Scholar
- Spielman A, Levine JF, Wilson ML. Vectorial capacity of North American Ixodes ticks. Yale J Biol Med. 1984;57:507–13.PubMedGoogle Scholar
- Walk ST, Xu G, Stull JW, Rich SM. Correlation between tick density and pathogen endemicity, New Hampshire. Emerg Infect Dis. 2009;15:585–7. DOIPubMedGoogle Scholar
- Ostfeld RS. Lyme disease: the ecology of a complex system. New York: Oxford University Press; 2011.
- Piesman J, Mather TN, Dammin GJ, Telford SR, Lastavica CC, Spielman A. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987;126:1187–9.PubMedGoogle Scholar
- Leiby DA. Transfusion-transmitted Babesia spp.: bull’s-eye on Babesia microti. Clin Microbiol Rev. 2011;24:14–28. DOIPubMedGoogle Scholar
- Healy GR, Spielman A, Gleason N. Human babesiosis: reservoir in infection on Nantucket Island. Science. 1976;192:479–80. DOIPubMedGoogle Scholar
- Spielman A, Etkind P, Piesman J, Ruebush TK II, Juranek DD, Jacobs MS. Reservoir hosts of human babesiosis on Nantucket Island. Am J Trop Med Hyg. 1981;30:560–5.PubMedGoogle Scholar
- Telford SR, Spielman A. Reservoir competence of white-footed mice for Babesia microti. J Med Entomol. 1993;30:223–7.PubMedGoogle Scholar
- Beck R, Vojta L, Curkovic S, Mrljak V, Margaletic J, Habrun B. Molecular survey of Babesia microti in wild rodents in central Croatia. Vector Borne Zoonotic Dis. 2011;11:81–3. DOIPubMedGoogle Scholar
- Duh D, Petrovec M, Trilar T, Avsic-Zupanc T. The molecular evidence of Babesia microti infection in small mammals collected in Slovenia. Parasitology. 2003;126:113–7. DOIPubMedGoogle Scholar
- Rar VA, Epikhina TI, Livanova NN, Panov VV. Genetic diversity of Babesia in Ixodes persulcatus and small mammals from North Ural and West Siberia, Russia. Parasitology. 2011;138:175–82. DOIPubMedGoogle Scholar
- Saito-Ito A, Kasahara M, Kasai M, Dantrakool A, Kawai A, Fujita H, Survey of Babesia microti infection in field rodents in Japan: records of the Kobe-type in new foci and findings of a new type related to the Otsu-type. Microbiol Immunol. 2007;51:15–24.PubMedGoogle Scholar
- Zamoto A, Tsuji M, Wei Q, Cho SH, Shin EH, Kim TS, Epizootiologic survey for Babesia microti among small wild mammals in northeastern Eurasia and a geographic diversity in the beta-tubulin gene sequences. J Vet Med Sci. 2004;66:785–92. DOIPubMedGoogle Scholar
- Zamoto A, Tsuji M, Kawabuchi T, Wei Q, Asakawa M, Ishihara C. US-type Babesia microti isolated from small wild mammals in eastern Hokkaido, Japan. J Vet Med Sci. 2004;66:919–26. DOIPubMedGoogle Scholar
- Telford SR, Mather TN, Adler GH, Spielman A. Short-tailed shrews as reservoirs of the agents of Lyme disease and human babesiosis. J Parasitol. 1990;76:681–3. DOIPubMedGoogle Scholar
- Birkenheuer AJ, Marr HS, Hladio N, Acton AE. Molecular evidence of prevalent dual piroplasma infections in North American raccoons (Procyon lotor). Parasitology. 2008;135:33–7. DOIPubMedGoogle Scholar
- Birkenheuer AJ, Horney B, Bailey M, Scott M, Sherbert B, Catto V, Babesia microti-like infections are prevalent in North American foxes. Vet Parasitol. 2010;172:179–82. DOIPubMedGoogle Scholar
- Tsuji M, Zamoto A, Kawabuchi T, Kataoka T, Nakajima R, Asakawa M, Babesia microti–like parasites detected in Eurasian red squirrels (Sciurus vulgaris orientis) in Hokkaido, Japan. J Vet Med Sci. 2006;68:643–6. DOIPubMedGoogle Scholar
- Bown KJ, Lambin X, Telford G, Heyder-Bruckner D, Ogden NH, Birtles RJ. The common shrew (Sorex araneus): a neglected host of tick-borne infections? Vector Borne Zoonotic Dis. 2011;11:947–53. DOIPubMedGoogle Scholar
- Franke J, Fritzsch J, Tomaso H, Straube E, Dorn W, Hildebrandt A. Coexistence of pathogens in host-seeking and feeding ticks within a single natural habitat in central Germany. Appl Environ Microbiol. 2010;76:6829–36. DOIPubMedGoogle Scholar
- Franke J, Meier F, Moldenhauer A, Straube E, Dorn W, Hildebrandt A. Established and emerging pathogens in Ixodes ricinus ticks collected from birds on a conservation island in the Baltic Sea. Med Vet Entomol. 2010;24:425–32. DOIPubMedGoogle Scholar
- Hildebrandt A, Franke J, Meier F, Sachse S, Dorn W, Straube E. The potential role of migratory birds in transmission cycles of Babesia spp., Anaplasma phagocytophilum, and Rickettsia spp. Ticks Tick Borne Dis. 2010;1:105–7. DOIPubMedGoogle Scholar
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–98. DOIPubMedGoogle Scholar
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A. 2003;100:567–71. DOIPubMedGoogle Scholar
- Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, Hosts as ecological traps for the vector of Lyme disease. Proc Biol Sci. 2009;276:3911–9. DOIPubMedGoogle Scholar
- Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. Climate, deer, rodents, and acorns as determinants of variation in Lyme disease risk. PLoS Biol. 2006;4:e145. DOIPubMedGoogle Scholar
- Goethert HK, Telford SR. What is Babesia microti? Parasitology. 2003;127:301–9. DOIPubMedGoogle Scholar
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.PubMedGoogle Scholar
- Nakajima R, Tsuji M, Oda K, Zamoto-Niikura A, Wei Q, Kawabuchi-Kurata T, Babesia microti–group parasites compared phylogenetically by complete sequencing of the CCTη gene in 36 isolates. J Vet Med Sci. 2009;71:55–68. DOIPubMedGoogle Scholar
- Goethert HK, Cook JA, Lance EW, Telford SR. Fay and Rausch 1969 revisited: Babesia microti in Alaskan small mammals. J Parasitol. 2006;92:826–31. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 18, Number 12—December 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Michelle H. Hersh, Department of Biology, Eastern Michigan University, 441 Mark Jefferson Science Complex, Ypsilanti, MI 48197, USA
Top