Volume 18, Number 8—August 2012
Dispatch
Seroprevalence and Cross-reactivity of Human Polyomavirus 9
Abstract
Many humans have antibodies against simian lymphotropic polyomavirus (LPyV), but its DNA has not been found in humans. Identification of human polyomavirus 9 (HPyV9) led us to compare the seroprevalence and cross-reactivity of LPyV and HpyV9. Results could indicate that humans who have antibodies against LPyV are infected by HPyV9.
To date, 9 human polyomaviruses (HPyVs) have been identified: BK polyomavirus, JC polyomavirus, Karolinska Institute polyomavirus, Washington University polyomavirus, human polyomavirus 6 and 7, Trichodysplasia spinulosa-associated polyomavirus, Merkel cell polyomavirus (MCPyV), and human polyomavirus 9 (HPyV9), which was identified in 2010 in human blood and skin samples (1,2). Serologic studies have shown that most adults have been exposed to HPyVs, and cross-reactivity studies have shown antigenic similarities between simian virus 40 and BK polyomavirus and, to a lesser extent, between simian virus 40 and JC polyomavirus (3–5). Interpretation of phylogenetic analysis of viral protein 1 (VP1) sequences predicts that cross-reactivity might also occur between Trichodysplasia spinulosa-associated polyomavirus and Bornean orangutan PyV, between MCPyV and chimpanzee polyomaviruses, and between HPyV9 and simian lymphotropic polyomavirus (LPyV).
Serologic survey results have shown that 15.0%–30.0% of humans have antibodies against LPyV, suggesting that the human population has been exposed to an antigenically related PyV (4–6). However, because LPyV DNA sequences have not been reported among humans (7,8), LPyV has thus far been considered to be a virus with a narrow host range, limited to nonhuman primates. The 2010 identification of HPyV9 (1,2), a virus phylogenetically related to LPyV (84.0% of identity), led us to investigate the seroprevalence of this newly discovered polyomavirus and to evaluate the existence of cross-reactivity that might explain the LPyV seroprevalence in humans.
We investigated the seroprevalence of HPyV9, MCPyV, and LPyV in children 1–14 years of age and adults18–85 years of age in the Ferrara region of Italy during December 2010–September 2011, and attempted to determine whether cross-reactivity between LPyV and HPyV9 might explain the latter’s seroprevalence reported among humans. Serum samples from 139 children (63 boys, 76 girls) and 186 adults (82 men, 104 women) were analyzed for HPyV9, LPyV, and MCPyV antibodies. The serum samples from healthy adult blood donors aged 18–65 years were obtained from the University Hospital of Ferrara Blood Center, and samples from children and from adults 66–85 years of age were obtained from the Clinical Analysis Laboratory, University Hospital of Ferrara, by using a protocol approved by the local county ethics committee at University of Ferrara. Consent from participants was not requested for polyomavirus testing; the identity of the sources was removed from the samples, and they were analyzed anonymously. To detect antibodies against HPyV9, we set up a virus-like particle (VLP)–based ELISA similar to the assay we developed for MCPyV (9). We obtained the HPyV9 VP1 coding sequence by total synthesis with a codon usage adapted for expression in Spodoptera frugiperda cells (Genscript, Piscataway, NJ, USA) (GenBank accession no. HQ696595). We used the Bac-to-Bac Baculovirus Expression System (Invitrogen, Fisher Scientific, Illkirch, France) to generate recombinant baculoviruses. HiFive cells maintained in Grace medium (Invitrogen) were infected with the different recombinant baculoviruses for the production of the different VLPs. VLPs were purified, and the presence of VLPs was determined by electron microscopy (9) (Technical Appendix Figure 1).
ELISAs were performed (9) in wells coated with 100 ng of VLPs. We determined concentration of HPyV9, LPyV, and MCPyV VLPs by using the Qubit Protein Assay Kit (Invitrogen). We used human serum samples diluted 1:100 and peroxidase-conjugated goat anti-human IgG (Clinisciences, Nanterre, France) diluted 1:20,000 to detect binding of human IgG. The cutoff values for all assays were set at 0.2, as determined previously for MCPyV and LPyV (9).
In all, 81 (24.9%) of the 325 investigated study participants were seropositive for HPyV9, 82 (25.2%) were positive for LPyV, and 252 (77.5%) were seropositive for MCPyV. Seventy-six HPyV9-positive participants were also LPyV positive, whereas the ELISA optical density was found to be close to the cutoff value for the remaining 11 discordant cases. We performed proportional analysis between groups, correlation analysis between HPyV9 and LPyV reactivity and between HPyV9 and MCPyV reactivity by using the χ2 test and the nonparametric Spearman test, respectively, using XLStat software (Addinsoft, Paris, France). The antibody reactivity of the 325 samples analyzed showed no correlation between HPyV9 and MCPyV ELISA results (ρ = 0.14, p = 0.0001; Figure 1, panel A) but a strong correlation between HPyV9 and LPyV ELISA results (ρ = 0.84, p = 0.001; Figure 1, panel B). In addition, antibody titers against HPyV9 and LPyV were determined for a subset of 15 human samples. As shown in Figure 1, panel C, the antibody titer against HPyV9 was always higher than the titer against LPyV (mean geometric antibody titer 2,660 against HPyV9 and 877 against LPyV).
To assess the degree of antigenic cross-reactivity between HPyV9 and LPyV, we performed competition assays by preincubation (1 h at 37°C) of the 15 anti-HPyV9–positive serum samples (diluted 1:100) by using 2 µg of HPyV9, LPyV, and MCPyV VLPs. In HPyV9 ELISA, the reactivity declined dramatically to undetectable levels after preincubation by HPyV9 VLPs but not by LPyV and MCPyV VLPs (Table). Similarly, the serum reactivity in LPyV ELISA declined to undetectable levels (or to low levels in 3 cases) after preincubation with both HPyV9 and LPyV but not with MCPyV VLPs. Dose-ranging competition experiments were performed on serum samples from 2 persons (Technical Appendix Figure 2), confirming the specificity of the inhibition. These results indicate that the HPyV9 reactivity detected in human serum samples was caused by HPyV9 infection and not by infection with the closely related LPyV. The findings also indicate that detection of LPyV antibodies in human serum samples should be attributed to cross-reacting HPyV9 antibodies.
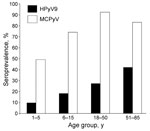
HPyV9 infection was confirmed in 27.5% of adults18–50 years of age and in 42.4% of adults 51–85 years of age (Figure 2). MCPyV infection was confirmed in 82.5% and 83.3%, respectively, of the same population groups. Among children 1–7 years of age, few HPyV9-positive subjects (10.1%) were detected compared with MCPyV-positive subjects (49.3%). HPyV9 and MCPyV antibodies were detected in 18.6% and 74.3% of children 7–14 years of age, respectively. No difference in HPyV9 seroprevalence was observed among children according to sex (14.3% vs. 14.5%, p = 0.98). Among adults, HPyV9 seroprevalence was higher among men than women (47.6% vs. 21.1%, p = 0.007). No difference in MCPyV antibodies according to sex was observed among children (69.8% vs. 55.3%, p = 0.39) or adults (86.6% vs. 91.3%, p = 0.80).
The HPyV9 antibodies detected in humans did not cross-react with MCPyV VLPs but cross-reacted strongly with LPyV VLPs in agreement with the recently published findings by Trusch et al., who used VP1 capsomers (10). Moreover, inhibition analysis suggested that LPyV does not infect humans or is limited in scope and does not circulate widely among humans.
Our finding that 61 (32.8%) of 186 adults were seropositive for HPyV9 supports the view that this virus is commonly circulating and infects humans throughout life. However, the HPyV9 seroprevalence was low compared with that observed for other human polyomaviruses (3,5,11–14), with the exception of that for human polyomavirus 7 (13).
Primary exposure for other HPyVs often occurs in early childhood, as found for MCPyV in this study and others (5,12). The detection of antibodies in 10.1% of children1–7 years of age and the increasing seroprevalence related to increasing age among adults suggests that infection by HPyV9 occurs at all ages. It is not surprising that HPyV9 seroprevalence is lower than the seroprevalence observed for other polyomaviruses because HPyV9 DNA has been detected in only a few persons (1,2), compared with the high frequency reported for other skin polyomaviruses (13,15). In conclusion, HPyV9 circulates widely in humans but not as commonly as other polyomaviruses. Further studies are needed to identify the clinical role of this polyomavirus.
Mr Nicol is enrolled in the PhD program in infectious diseases at the University François Rabelais, Tours, France. His research is focused on new skin polyomaviruses.
Acknowledgments
We thank Pierre-Yves Sizaret and Julien Gaillard for help with electron microscopy of the VLPs.
J.N. was supported by a doctoral grant from the Région Centre, France, and E.M. is a postdoctorate Fellow at the University of Ferrara. This study was also funded by grants to P.C. from the “Ligue Contre le Cancer” (Comité du Cher and Comité de l’Indre, 2010) and to M.T. from the University of Ferrara.
References
- Scuda N, Hofmann J, Calvignac-Spencer S, Ruprecht K, Liman P, Kühn J, A novel human polyomavirus closely related to the African green monkey–derived lymphotropic polyomavirus. J Virol. 2011;85:4586–90. DOIPubMedGoogle Scholar
- Sauvage V, Foulongne V, Cheval J, Ar Gouilh M, Pariente K, Dereure O, Human polyomavirus related to African green monkey lymphotropic polyomavirus. Emerg Infect Dis. 2011;17:1364–70.PubMedGoogle Scholar
- Carter JJ, Madeleine MM, Wipf GC, Garcea RL, Pipkin PA, Minor PD, Lack of serologic evidence for prevalent simian virus 40 infection in humans. J Natl Cancer Inst. 2003;95:1522–30. DOIPubMedGoogle Scholar
- Viscidi RP, Clayman B. Serological cross reactivity between polyomavirus capsids. Adv Exp Med Biol. 2006;577:73–84. DOIPubMedGoogle Scholar
- Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. DOIPubMedGoogle Scholar
- Takemoto KK, Furuno A, Kato K, Yoshiike K. Biological and biochemical studies of African green monkey lymphotropic papovavirus. J Virol. 1982;42:502–9.PubMedGoogle Scholar
- Focosi D, Maggi F, Andreoli E, Lanini L, Ceccherini-Nelli L, Petrini M. Polyomaviruses other than JCV are not detected in progressive multifocal leukoencephalopathy. J Clin Virol. 2009;45:161–2. DOIPubMedGoogle Scholar
- Costa C, Bergallo M, Terlizzi ME, Cavallo GP, Cavalla P, Cavallo R. Lack of detection of lymphotropic polyomavirus DNA in different clinical specimens. J Clin Virol. 2011;51:148–9. DOIPubMedGoogle Scholar
- Touzé A, Gaitan J, Arnold F, Cazal R, Fleury MJ, Combelas N, Generation of Merkel cell polyomavirus (MCPyV)–like particles and their application to detection of MCPyV antibodies. J Clin Microbiol. 2010;48:1767–70. DOIPubMedGoogle Scholar
- Trusch F, Klein M, Finsterbusch T, Kühn J, Hofmann J, Ehlers B. Seroprevalence of human 23 polyomavirus 9 and cross-reactivity to African green monkey–derived lymphotropic 24 polyomavirus. J Gen Virol. 2012;93:698–705. DOIPubMedGoogle Scholar
- Nguyen NL, Le BM, Wang D. Serologic evidence of frequent human infection with WU and KI polyomaviruses. Emerg Infect Dis. 2009;15:1199–205. DOIPubMedGoogle Scholar
- Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250–6. DOIPubMedGoogle Scholar
- Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509–15. DOIPubMedGoogle Scholar
- van der Meijden E, Kazem S, Burgers MM, Janssens R, Bouwes Bavinck JN, de Melker H, Seroprevalence of trichodysplasia spinulosa–associated polyomavirus. Emerg Infect Dis. 2011;17:1355–63.PubMedGoogle Scholar
- Foulongne V, Kluger N, Dereure O, Mercier G, Molès JP, Guillot B, Merkel cell polyomavirus in cutaneous swabs. Emerg Infect Dis. 2010;16:685–7.PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 18, Number 8—August 2012
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Pierre Coursaget, Faculté des Sciences Pharmaceutiques, 31 Ave Monge, 37200 Tours, France
Top