Volume 19, Number 11—November 2013
Dispatch
Incidence of Influenza A(H1N1)pdm09 Infection, United Kingdom, 2009–2011
Abstract
We conducted a longitudinal community cohort study of healthy adults in the UK. We found significantly higher incidence of influenza A(H1N1)pdm09 infection in 2010–11 than in 2009–10, a substantial proportion of subclinical infection, and higher risk for infection during 2010–11 among persons with lower preinfection antibody titers.
Case-based population-level surveillance and cross-sectional serologic surveys to estimate incidence and patterns of influenza infection are limited by the lack of accurate denominator data, inability to account for subclinical infections, difficulties in distinguishing between antibodies induced by natural infection and vaccination, and use of samples from high-risk groups. For these reasons, community-based longitudinal studies are ideal to estimate the incidence of infection and spectrum of illness. However, studies of this design describing the 2009 pandemic of influenza A(H1N1)pdm09, reported only from Hong Kong, Singapore, and Vietnam, examine only the 2009–10 season (1–3).
The epidemiology of A(H1N1)pdm09 in the United Kingdom during 2009–2011 was characterized by 3 distinct waves: first wave, April–August 2009; second wave, September 2009–April 2010; and third wave, August 2010–April 2011. We report results from a community-based longitudinal cohort study that compared the epidemiology of influenza A(H1N1)pdm09 infection over the second and third waves. The North West London Research Ethics Committee approved this study (reference 09/H0724/27).
A total of 342 healthy adult staff and students of Imperial College London (London, UK) were recruited during September–November 2009 and followed for 2 consecutive influenza seasons: 2009–10 and 2010–11 (Figure 1). Participants’ median age was 28 years (interquartile range 20–36 years); 83% were <40 years of age. At each time point, collected serum samples were tested for antibodies to A(H1N1)pdm09 virus (A/England/195/09 strain) by the hemagglutination-inhibition (HI) assay (4). Participants were asked to record temperature, self-sample, and return nasal swabs when experiencing influenza-like symptoms. Swabs were tested for respiratory viruses with standardized real-time reverse transcription PCR. Influenza seroprevalence rates were defined as the proportion of persons with HI titers >32 (4).
Because our study began at the end of the first pandemic wave, cumulative incidence of A(H1N1)pdm09 infection over the first wave was estimated as the difference between age-specific seroprevalence rates at recruitment (T0 in Figure 1) and published prepandemic (2008) seroprevalence rates for England (4). Incident infection was defined as antibody seroconversion (4-fold rise in HI titer) in paired serum samples collected at the start and end of a wave among unvaccinated persons (because HI assay cannot differentiate infection from vaccination) or detection of A(H1N1)pdm09 virus in nasal swabs. The incidence of infection was estimated for the second and third waves as the proportion of incident infections among unvaccinated participants.
Development of any symptoms was recorded on a Web-based questionnaire emailed to participants every 3 weeks. The average response rate was 75%. Illness episodes were categorized as acute respiratory infection (episode with any symptoms), influenza-like illness ([ILI] episode with fever plus cough or sore throat), and fever (recorded temperature >38°C) alone. Visits to primary care or hospital during illness were also recorded. Data were analyzed using Stata version 9.0 (StataCorp, College Station, TX, USA) with the χ2 test to compare proportions and t test to compare means after checking for normal distribution by assessing for kurtosis, skewness, and the Shapiro-Wilk test. Hosmer-Lemeshow test was used to estimate goodness-of-fit for each logistic regression.
At recruitment, after the first pandemic wave, A(H1N1)pdm09 seroprevalence was 26% (95% CI 21.4–31.2), with seroprevalence significantly higher in participants 18–25 years of age than in older age groups (Table 1). Participants with ILI in the preceding 3 months corresponding to the first wave had significantly higher (p<0.001) mean A(H1N1)pdm09 virus HI titers, which in conjunction with the age distribution, suggests first-wave infection rather than cross-reactive antibodies (5). Overall cumulative incidence during the first wave was 12.7% (95% CI 7.1%–18.4%) and 26.6% (95% CI 15.3%–37.8%) among participants 18–25 years of age with no increase in older age groups (Technical Appendix Table 1).
The incidence of infection over the third pandemic wave was significantly higher (p = 0.02) than over the second wave (Figure 1). Among participants with prewave titers <8, the incidence of infection was significantly higher over the third wave than over the second wave (p<0.001); incidence did not differ for participants with prewave titers >8 (Table 2, Appendix). Age-specific incidence was significantly higher (p = 0.01) over the third wave than the second wave among participants 26–40 years of age (third wave: 25.4% [95% CI 15.2–35.5]; second wave: 10.9% [95% CI 5.1–16.7]) but not the other age groups (Table 2, Appendix). For 11 infected participants with paired serum samples and virus detected in nasal swabs, 2 (18%) did not show antibody seroconversion (Technical Appendix Table 2).
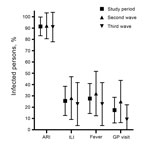
During an illness episode, 20% of infected participants reported fever or ILI, 17% visited their general practitioner, and none visited a hospital (Figure 2). Because predictions of a small third pandemic wave were disproved (4), the reasons for this large wave remained unclear. Multivariate logistic regression was undertaken with infection as the dependent variable and age, sex, and prewave titers as independent variables. Each doubling increase in prewave HI titers, after adjustment for age and sex, was associated with significantly lower risk for infection (odds ratio 0.92, 95% CI 0.9–1.0, p = 0.04) during the third, but not the second, wave (Table 2, Appendix).
Incidence of A(H1N1)pdm09 infection was significantly higher among healthy adults during the third pandemic wave (2010–11) than during the second wave (2009–10). This study complements and corroborates clinical surveillance data and population-sampling seroepidemiology from the United Kingdom (4,6,7), United States (8) and elsewhere (9).
The reasons for this unexpectedly larger third wave in the postpandemic season remain unclear. We show an increased risk for A(H1N1)pdm09 infection associated with lower antibody levels at the start of the season, irrespective of age, during the third, but not the second, wave. Because no substantial viral genetic change occurred between the waves (7), our finding suggests that the third wave was driven by infection among susceptible persons remaining antibody-naive at the end of the second wave. This thesis is supported by serosurveillance data showing lower infection rates over the third wave among age groups with the highest infection rates over previous pandemic waves (7,8). Our interpretation is further strengthened by a meta-analysis of serologic data from 19 countries that showed 20%–27% incidence of infection during the first pandemic year, suggestive of a large population susceptible to infection in subsequent seasons (10).
Incidence in our cohort was lower than that estimated for England by cross-sectional serosurveys (7,11). This finding may reflect our accounting for individual-level vaccination status and baseline antibody titers; data usually unobtainable with cross-sectional population-sample serosurveys. However, our study did not include children or elderly persons, which limits the generalizability of our findings. A major advantage of longitudinal cohort studies recording clinical data is identification of subclinical and asymptomatic infections. More than 80% of participants did not seek primary care or have surveillance-defined ILI indicating a high proportion of subclinical infection among healthy adults undetectable by routine case-based surveillance. We also describe persons shedding virus without antibody seroconversion, a phenomenon recently reported in Vietnam and the United Kingdom (4,12). Although these nonseroconverters might have antibodies detectable by microneutralization assay, such nonseroconverters, undetectable by serosurveys using the standard HI assay, further highlight the possibility of underestimating community infection rates when cross-sectional serosurveys alone are used.
Despite our intensive symptom ascertainment, 4 participants with influenza reported no symptoms. Cross-reactive cellular immune responses that are highly prevalent in the population (13) have recently been shown to be associated with protection against symptomatic illness (14).
Our analysis of pandemic influenza in a community cohort over successive seasons offers insight into contributors of the unexpectedly larger third pandemic wave. Our analysis also highlights the necessity of using cohorts to complement routine case-based surveillance to estimate influenza burden.
Dr Sridhar is a postdoctoral researcher at the Department of Respiratory Medicine, Imperial College London. His research interests encompass the immune epidemiology of influenza and tuberculosis and the development and evaluation of vaccination strategies against respiratory pathogens.
Acknowledgments
We thank all willing participants of this study. We express our grateful appreciation to Marie Bautista, Sharleen Bowes, Bianca Fortunaso, and Sharna Lloyd-James for processing all the samples and our clinical research nurses.
A.L is a Wellcome Trust Senior Research Fellow in Clinical Science and NIHR Senior Clinical Investigator, S.S. is supported by the Imperial College Healthcare NHS Trust, and S.B. is supported by a Medical Research Council funded PhD studentship.
References
- Chen MI, Lee VJ, Barr I, Lin C, Goh R, Lee C, Risk factors for pandemic (H1N1) 2009 virus seroconversion among hospital staff, Singapore. Emerg Infect Dis. 2010;16:1554–61. DOIPubMedGoogle Scholar
- Riley S, Kwok KO, Wu KM, Ning DY, Cowling BJ, Wu JT, Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a longitudinal community cohort study. PLoS Med. 2011;8:e1000442. DOIPubMedGoogle Scholar
- Horby P. Mai le Q, Fox A, Thai PQ, Thi Thu Yen N, Thanh le T, et al. The epidemiology of interpandemic and pandemic influenza in Vietnam, 2007–2010: the Ha Nam household cohort study I. Am J Epidemiol. 2012;175:1062–74.
- Hardelid P, Andrews NJ, Hoschler K, Stanford E, Baguelin M, Waight PA, Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009. Health Technol Assess. 2010;14:115–92 .PubMedGoogle Scholar
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. DOIPubMedGoogle Scholar
- Adamson WE, McGregor EC, Kavanagh K, McMenamin J, McDonagh S, Molyneaux PJ, Population exposure to a novel influenza A virus over three waves of infection. J Clin Virol. 2011;52:300–3. DOIPubMedGoogle Scholar
- Hoschler K, Thompson C, Andrews N, Galiano M, Pebody R, Ellis J, Seroprevalence of influenza A(H1N1)pdm09 virus antibody, England, 2010 and 2011. Emerg Infect Dis. 2012;18:1894–7. DOIPubMedGoogle Scholar
- Ross TM, Hairong L, Chia BS, Hill E, Weirback H, Zimmer S. Prevalence of antibodies against seasonal influenza A and B viruses during the 2009–2010 and 2010–2011 influenza seasons in residents of Pittsburgh, PA, USA. PLoS Curr. 2011;3:RRN1265. DOIPubMedGoogle Scholar
- Orsted I, Molvadgaard M, Nielsen HL, Nielsen H. The first, second and third wave of pandemic influenza A (H1N1)pdm09 in North Denmark Region 2009–2011: a population-based study of hospitalizations. Influenza Other Respi Viruses. 2013;7:776–82 . DOIPubMedGoogle Scholar
- Van Kerkhove MD, Hirve S, Koukounari A, Mounts AW. Estimating age-specific cumulative incidence for the 2009 influenza pandemic: a meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respi Viruses. 2013;7:872–86. DOIPubMedGoogle Scholar
- Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–8. DOIPubMedGoogle Scholar
- Cauchemez S, Horby P, Fox A. Mai le Q, Thanh le T, Thai PQ, et al. Influenza infection rates, measurement errors and the interpretation of paired serology. PLoS Pathog. 2012;8:e1003061.
- Sridhar S, Begom S, Bermingham A, Ziegler T, Roberts KL, Barclay WS, Predominance of heterosubtypic IFN-gamma-only-secreting effector memory T cells in pandemic H1N1 naive adults. Eur J Immunol. 2012;42:2913–24 . DOIPubMedGoogle Scholar
- 14. Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19:1305–12.
Figures
Tables
Cite This ArticleTable of Contents – Volume 19, Number 11—November 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Saranya Sridhar, Department of Respiratory Medicine, Imperial College London, St. Mary’s Campus, Bldg 2, Norfolk Pl, London W2 1PG, UK
Top