Volume 2, Number 3—July 1996
Synopsis
Coccidioidomycosis: A Reemerging Infectious Disease
Abstract
Coccidioides immitis, the primary pathogenic fungus that causes coccidioidomycosis, is most commonly found in the deserts of the southwestern United States and Central and South America. During the early 1990s, the incidence of coccidioidomycosis in California increased dramatically. Even though most infections are subclinical or self-limited, the outbreak is estimated to have cost more than $66 million in direct medical expenses and time lost from work in Kern County, California, alone. In addition to the financial loss, this pathogen causes serious and life-threatening disseminated infections, especially among the immunosuppressed, including AIDS patients. This article discusses factors that may be responsible for the increased incidence of coccidioidomycosis (e.g., climatic and demographic changes and the clinical problems of coccidioidomycosis in the immunocompromised) and new approaches to therapy and prevention.
Emerging infectious diseases have been defined as “infections that have newly existed in a population or have existed but are rapidly increasing in incidence or geographic range” (1). In what sense is coccidioidomycosis an emerging infectious disease? Coccidioidomycosis is not a new disease; it was first recognized and reported slightly more than 100 years ago by a medical student in Argentina (2). In fact, coccidioidomycosis has affected inhabitants of the desert Southwest for thousands of years (3). However, in the past several years, the number of cases of coccidioidomycosis has increased dramatically, and the clinical symptoms of this illness have changed in patients with acquired immunodefficiency syndrome (AIDS). In this article, we explore some of the reasons for the increased incidence of coccidioidomycosis, review the new clinical data, and discuss current approaches to therapy and prevention.
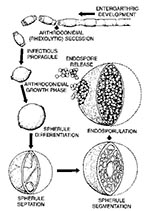
Coccidioidomycosis is caused by Coccidioides immitis, a dimorphic fungus that grows as a mold in the soil. The mold forms arthroconidia within the hypha, a type of conidia formation known as enteroarthric development (Figure 1) (4). C. immitis is the only species within the primary pathogenic fungi that has this type of conidia development. Alternate conidia undergo autolysis, leaving empty spaces between viable arthroconidia. The arthroconidia are released into the atmosphere when the wind ruptures the hypha. C. immitis infects humans and animals almost exclusively by the respiratory route (5). Once inhaled, the arthroconidia cluster in the lungs and undergo a dramatic morphologic change. The round cells, which develop into spherules, undergo repeated internal divisions until they are filled with hundreds to thousands of offspring, termed endospores. This process occurs over 48 to 72 hours (6). When the spherule ruptures, each released endospore has the capacity to develop into a mature spherule.
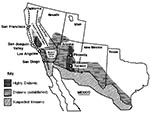
C. immitis is primarily found in desert soil. It is present in highest numbers in the San Joaquin Valley in California, southern Arizona, southern New Mexico, west Texas, and the desert areas of northern Mexico (Figure 2). The organism is also found in scattered foci in coastal southern California, southern Nevada, and Utah (7) and is endemic in a few areas in Central and South America, especially in Venezuela (7). C. immitis is distributed unevenly in the soil and seems to be concentrated around animal burrows and ancient Indian burial sites (8,9); it is usually found 4 to 12 inches below the surface of the soil (7).
Since C. immitis infects humans by the respiratory route, exposure to dust is one critical factor determining the risk for infection (10). Coccidioidomycosis is not spread from person to person, except in extraordinary circumstances. Coccidioidomycosis probably had its most profound effect on the population of the United States during World War II when several training airfields were built in the San Joaquin Valley. The rate of new infections in military personnel was 8% to 25% per year (10). Coccidioidomycosis was the most common cause of hospitalization at many airbases in the Southwest. Though the death rate was very low, many soldiers were sick for weeks to months, and their training was completely disrupted. At least in part because of efforts to minimize dust, the infection rate declined as the war went on (10).
The incidence of coccidioidomycosis varies with the season; it is highest in late summer and early fall when the soil is dry and the crops are harvested (10). If it rains at this time of the year (which is unusual in southern California), disease incidence declines as the amount of dust decreases. Dust storms are frequently followed by outbreaks of coccidioidomycosis. One particularly severe dust storm in 1977 carried dust from the San Joaquin Valley up to the San Francisco Bay area and resulted in hundreds of cases of nonendemic coccidioidomycosis in areas north of the San Joaquin Valley (11). More recently, an earthquake centered in Northridge, California, was associated with 170 cases of acute coccidioidomycosis in Ventura County, which normally has a low incidence of this disease. The airborne dust associated with landslides triggered by the earthquake was implicated in the increase in the number of cases (12).
Occupational or recreational exposure to dust is also an important consideration. Agricultural workers, construction workers, or others (such as archeologists) who dig in the soil in the disease-endemic area are at increased risk for the disease (13,14). During World War II, C. E. Smith, one of the most perceptive and influential epidemiologists to study coccidioidomycosis, recommended dust control as a primary measure to reduce risk for exposure (10). However, because the desert is inherently dusty, many cases of coccidioidomycosis are acquired just by driving through the disease-endemic area.
C. immitis is transmitted by the respiratory route. Smith et al., in a prospective study of cases of coccidioidomycosis acquired during World War II by soldiers at three San Joaquin Valley airbases, skin-tested the airmen periodically and questioned them about illnesses in the interval. They found that most infections (60%) were asymptomatic and resolved spontaneously; 15% were not severe enough to require medical care, and 25% were clinically important and required a substantial amount of time off work (15). In symptomatic patients, the pulmonary illness ranges from a self-limited flulike illness to pneumonia (16). Approximately 5% of primary infections result in erythema nodosum or erythema marginatum with associated noninfectious arthritis; most of those patients have a self-limited infection (17). Particularly in persons with diabetes, multiple thin-walled chronic cavities tend to develop as a residual effect of pulmonary coccidioidomycosis (18). Unlike in tuberculosis, in coccidioidomycosis, dissemination almost always becomes evident within a few weeks of the primary pneumonia, although in cases of limited dissemination it may not become clinically evident until months later (15,19). Cocidioidomycosis can disseminate and cause miliary disease, bone and joint infection, skin disease, soft tissue abscesses, and meningitis (15,16). These extrapulmonary complications are uncommon (<5% of infections).
The risk for disseminated coccidioidomycosis is much higher among some ethnic groups, particularly African-Americans and Filipinos. In these ethnic groups, the risk for disseminated coccidioidomycosis is tenfold that of the general population (5,20). Presumably, a gene (or genes) that increases susceptibility to infection is more prevalent in these ethnic groups than in the general population. Such a resistance gene has been identified in mice (21-23), but not yet in humans. The mechanism by which the resistance genes affect the course of the disease in mice is not clear. Pregnant women and the immunosuppressed are also at high risk for developing disseminated disease (Figure 3) (24). One study demonstrated that the growth rate of spherules was influenced by human sex hormones, which may partially account for the increased risk of disseminated disease in pregnancy (25). Pregnancy also redirects the immune response toward humeral (TH2) immunity and away from delayed hypersensitivity (TH1) (26), which may influence resolution of coccidioidomycosis. Generalized suppression of cell mediated immunity also increases the risk of disseminated disease (27). Coccidioidomycosis is particularly severe in patients with organ transplants or AIDS.
Though disseminated coccidioidomycosis is uncommon, and symptomatic coccidioidal pneumonia usually resolves without therapy, many of these patients are very ill for weeks to months. Galgiani reported that a group of college students in Tucson who had coccidioidomycosis required an average of six clinic visits before the disease resolved (16). Therefore, this can be an expensive illness in terms of medical costs and time lost from work or school, even when the infection resolves spontaneously.
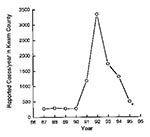
Kern County, in the San Joaquin Valley, California, is one of the most highly coccidioidomycosis-endemic regions. The number of new cases of coccidioidomycosis in the area has varied widely from year to year; a low incidence of coccidioidomycosis from 1987 to 1990 (<500 reported cases a year in Kern County), was followed by a high incidence from 1991 to 1994 (28-30). The number of reported cases, which were identified by serologic testing at the Kern County Health Department (the reference serology laboratory for the county), probably represent approximately 10% of the total number of infected persons in that county (Figure 4) (28). The medical costs for infected persons in Kern County are estimated at $66 million (29). In 1992, 4,500 new cases were reported to the California State Department of Health Services (30), most from Kern County; the number of new cases also increased in almost all counties in central and southern California (30). The increase in reported cases in California in 199192 was dramatic but certainly an underestimate of the magnitude of the problem (31).
The epidemic seems to be waning, for reasons that are not clear, but the marked increase in incidence from the 1980s to 1991 through 1993 is indisputable. What factors may account for this increase? One major consideration is the weather. C. E. Smith observed years ago that the number of cases of coccidioidomycosis was higher in the summer after a rainy winter than after a dry winter (10). In March 1991, a 5-year drought in California ended with a heavy rainfall. Rainfall was also heavy in the winters of 1992 and 1993. Though the relationship between the weather and the density of C. immitis in the soil may never be understood in detail, the following scenario seems plausible. During drought years, the number of organisms competing with C. immitis decreases. C. immitis does not thrive, but it remains viable though dormant. After heavy rain, the arthrocondia germinate and multiply to a higher density than usual because of the lack of competing organisms. Once the soil dries in the late summer and fall, the arthroconidia become airborne and potentially infectious (29).
Another reason for the sudden increase in disease incidence might have been the number of susceptible persons in the disease-endemic area. The number may have been the result of both increased migration of susceptible persons and decreased immunity in the indigenous population. Immunity comes from prior infection and is manifest as a positive coccidioidin skin test. In almost all cases, coccidioidomycosis confers lifelong immunity. As a result of years of low incidence, the number of nonimmune persons may have increased, as evidenced by the decrease in prevalence of positive coccidioidin skin tests among local high school students. In 1939, 50% to 60% of high school students in the San Joaquin Valley had positive skin tests (17), but in the 1980s only 3% to 5% of high school students had positive skin tests (T. Larwood, pers. comm.). Given the historical data, this estimate seems low, but another study also found a low prevalence. In 1985, workers in Tucson estimated that 30% of a random sample of persons in a Hispanic neighborhood had positive skin tests (32). In addition to the drought, irrigation of fields, the increasing amount of land under cultivation, and a decrease in indoor dust due to the widespread use of air conditioning may also have played a role in the relatively low incidence of infections in the 1980s.
C. immitis is a primary pathogen that can cause disease in immunologically healthy persons. In the population as a whole, fewer than 5% of infected persons have persistent pulmonary infection or extrapulmonary dissemination of the disease (16). The incidence of clinically significant disease in immunosuppressed patients is much higher. In one study symptomatic coccidioidomycosis developed in 18 (7%) of 260 renal transplant patients in Arizona over a 10-year period, primarily in the first year after transplantation (33). This rate was substantially higher than the rate of infection in patients who were undergoing hemodialysis. Approximately 12 (67%) of infections in the patients with renal transplants were disseminated; the remainder were confined to the lung. Of patients with disseminated disease, 10 (83%) died, despite intensive therapy with amphotericin B. In another study from Tucson, all confirmed cases of coccidioidomycosis during a 4-year period were reviewed. The dissemination rate was 8 (73%) of 11 of patients who were receiving immunosuppressive therapies, compared with only 15 (14%) of 110 healthy controls (34). As more patients in the disease-endemic area receive liver, lung, and heart transplants, this problem will increase.
Pregnant women, especially those in the third trimester, are at high risk for developing disseminated coccidioidomycosis if they become infected (24). In the first and second trimesters, the risk is much lower. The reason is not entirely clear, but two factors may play a role: 1) the high sex hormone levels found in late pregnancy enhance the growth of C. immitis in vitro (25), and 2) the shift in the T-cell immune response late in pregnancy toward TH2 cytokines (26) interferes with resolving the infection. In experimental animals, pregnancy increases the severity of leishmaniasis, another infection hat is controlled by a TH1 T-cell response (35).
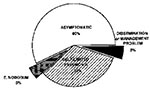
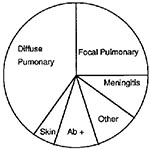
Coccidioidomycosis in AIDS patients is also very likely to be life-threatening. The first cases of coccidioidomycosis described in AIDS patients were atypical, with a reticulonodular chest x-ray pattern, positive blood cultures, and infection of multiple organs (36). As we have gained more experience with coccidioidomycosis in HIV-infected persons, we have learned that the clinical spectrum is broader than originally reported. Fish and his colleagues collected data from 77 AIDS patients with coccidioidomycosis who were treated by physicians in Arizona and California (37). They grouped the patients according to their clinical symptoms (Figure 5).
Although the largest group of patients had diffuse pulmonary infiltrates, a significant fraction had focal pulmonary disease, meningitis, or other extrapulmonary disease. Six patients had only a positive serologic test with no other evidence of infection. Excluding the patients who had only a positive serologic test, 81% of the patients in this series had a positive serologic test for coccidioidomycosis, either for IgM or IgG antibodies. However, only 69% of patients with diffuse pulmonary disease had a positive serologic test, and the death rate in this group was also the highest (70%). In all clinical groups, death was correlated with the number of circulating CD4 T-cells at the time of diagnosis. For clinicians, however, the most important message from this study is that coccidioidomycosis is not a uniformly fatal complication in patients with AIDS, and that many forms of this disease, including meningitis, respond to therapy. Patients with <200 CD4 T-cells/µl are more likely to have severe, disseminated infections.
A more recent prospective study of 170 HIV-infected persons in an area of Arizona where coccidioidomycosis is endemic showed a cumulative incidence of coccidioidomycosis of 25% over 41 months (38). The most important risk factors were the level of CD4 T-cells and the diagnosis of AIDS (as opposed to HIV infection). HIV-infected patients with AIDS or <250 CD4 T-cells/µl were 8 to 35 times more likely to get coccidioidomycosis. History of coccidioidomycosis, a history of a positive skin test for coccidioidomycosis, or a prolonged stay in the disease-endemic area were not associated with an increased risk for infection. These data suggest that most cases were primary infections in severely immunosuppressed patients. Since patients with AIDS were not more likely to be exposed to the spores of C. immitis and all patients were seen prospectively at 4-month intervals and tested for antibody to C. immitis, severe immunosuppression appeared to increase their risk for infection, as well as disease. As in the retrospective study reviewed above, the clinical symptoms varied widely, ranging from mild to extremely severe. Only one patient had antibody titers to C. immitis by complement fixation test without any other evidence of disease.
Various drugs are now available for treating coccidioidomycosis. In addition to amphotericin B, which must be given intravenously and is considerably toxic, triazole compounds have been found to be active agents for treating most manifestations of coccidioidomycosis. Fluconazole, in an uncontrolled study, was reported to be effective primary therapy for coccidioidal meningitis; since untreated coccidioidal meningitis is uniformly fatal, robust conclusions could be drawn from this trial (39). Forty-seven consecutive patients were treated with 400 mg/day of fluconazole; during the first 6 months of therapy, 33 (70%) of the patients responded to therapy. (A response was defined as a 40% reduction in a score, on the basis of clinical measurements and cerebrospinal fluid findings.) Two patients who did not respond to therapy died of coccidioidomycosis; both were HIV-positive. Because of previous experience with high relapse rates when azole therapy is stopped, the authors recommended lifelong treatment with fluconazole. In a small study, four of five patients treated for meningitis with itraconazole as sole therapy responded favorably (40). A recent article emphasized the high relapse rate after azole therapy is stopped (41). The alternative treatment to the azoles is amphotericin B. If amphotericin B is used to treat meningitis, however, it must be given intrathecally as well as intravenously, and this greatly increases the risk for a toxic reaction to that drug.
Clearly, fluconazole and itraconazole can be used to treat patients with nonmeningeal coccidioidomycosis (42-44). Whether one of these drugs is superior to the other, or how either one compares to amphotericin B is not known. It seems prudent to treat extremely ill patients with amphotericin B, at least until their clinical situation stabilizes, although no published studies support that point of view. However, few (if any) patients with the acute miliary form of coccidioidomycosis have been included in any of the reported studies of any of the azole drugs. New agents that are more active against coccidioidomycosis are still sorely needed.
Simple environmental measures, such as planting grass or paving roads in highly populated areas, decrease the amount of airborne dust and lower the risk for coccidioidomycosis (10). These measures do not necessarily eradicate C. immitis from the soil but lower the risk for airborne dispersion of the organism. At present, no practical method exists for eliminating C. immitis from the soil.
An alternative approach is to vaccinate persons at risk. A vaccine is feasible because natural infection almost always confers lifelong immunity from reinfection. Furthermore, good animal models exist to test vaccine candidates (21). Finally, genetically susceptible mice can be successfully immunized, which suggests that the genetically susceptible human population would also benefit from vaccination (21).
One vaccine that has been tested is a killed spherule vaccine developed by Pappagianis and Levine. It protected mice and other animals from experimental infection with C. immitis (45). Between 1980 and 1985, a double-blinded human study compared results of a formalin-killed spherule vaccine with results obtained from a placebo. In this study, which involved almost 3,000 people, only a minority of the vaccinated persons had positive skin test results to C. immitis. Although the incidence of coccidioidomycosis was low while this study was conducted, no difference was found in the number of cases of coccidioidomycosis or the severity of the disease in the vaccinated group compared with that for the placebo-receiving control group (46). One explanation for the ineffectiveness of this vaccine may be that relatively small numbers of killed organisms could be injected into human without unacceptable local side effects of pain and swelling. Nevertheless, the vaccine trial made it clear that immunization with tolerable numbers of whole killed-spherules does not provide immunoprotection against coccidioidomycosis in humans.
Since the cell wall of C. immitis is made up primarily of nonprotein macromolecules, it contains a large amount of material presumably nonantigenic for T lymphocytes. Therefore, the whole organism is not the ideal vaccine candidate. Ideally, one would like to vaccinate patients selectively only with antigens that stimulate a protective T-cell-mediated immune response. These antigens have been difficult to identify, and a consensus on what they are does not exist. Various approaches have been used to obtain antigenic proteins. In one, a lysate of arthroconidia (coccidioidin) or spherules (spherulin) was made (47). Alkali treatment has also been used to extract antigens from arthroconidia and spherules (48). Another approach has been to use C. immitis antigens obtained without extraction or autolysis. The advantage of this method is that one should obtain reproducible preparations of intact proteins. Cole and co-workers (49) found that when the outer conidial wall was removed from arthroconidia, the organism released various proteins (called the soluble conidial wall fraction). This mixture of proteins was extraordinarily effective in stimulating the proliferation of C. immitis-immune T cells in mice. Another antigenic mixture is a membranous material consisting primarily of proteins and lipids that the spherule phase of the organism spontaneously releases (the spherule outer wall). This spherule wall fraction has been shown to be an active antigen in T-cell-mediated immune responses in mice (50).
All of these mixtures are heterogeneous and difficult to fractionate biochemically. This is probably due, at least in part, to differences in glycosylation, which makes physically separating the proteins difficult. To resolve this problem, Galgiani and his colleagues deglycosylated the proteins from a toluene spherule lysate by using hydrogen fluoride (51,52). Although this treatment does remove all sugars, it is extraordinarily harsh and yields less than 10% of the initial protein, with most of the protein forming an insoluble precipitate. Nevertheless, the resulting product reacts with reference antiserum to C. immitis in immunoelectrophoresis. This antigen also stimulated a proliferative T-cell response in patient lymphocytes but not in those of the control group (noninfected donors).
Another way to attack the problem of generating pure C. immitis antigens is to use molecular biologic techniques. The advantage to this approach is that once antigens are molecularly cloned, and the protein is expressed, an essentially unlimited source of completely defined antigen is available. Therefore, one would not have to repeatedly grow C. immitis, extract the antigen, and purify it from a complex mixture. In addition, with the molecular approach, antigens could be delivered as part of a living vaccine system, should that be required to effectively immunize people against coccidioidomycosis. We believe that systematically identifying and evaluating C. immitis T-cell reactive antigens in experimental animals is a rational approach to the ultimate development of a vaccine. Our laboratories, in collaboration with Garry Cole, have used a murine T-cell line that is specific for soluble conidial wall fraction antigens to identify one cloned fragment of a C. immitis protein (53). Recently, genomic DNA clones coding for this protein have been identified and sequenced. Significant homology exists between this C. immitis antigen and the human enzyme 4 hydroxyphenylpyruvate dioxygenase (54). This protein has been expressed in bacteria and was found to elicit T-lymphocyte proliferative responses in mice immune to C. immitis. We are testing its efficacy as an experimental vaccine.
With the exception of alkali extracted spherules (55) and whole killed spherules (45), none of the T-cell reactive antigens have been shown to be immunoprotective in experimental models. However, it is reasonable to expect that some antigen, or mixture of antigens, will be found that can confer protective immunity in experimental animals. Molecular strategies are available to accomplish this task and are an important area of future research. Once a vaccine has been successfully tested in animals, another human vaccine trial would be feasible.
Dr. Kirkland is associate professor of pathology and medicine; Dr. Fierer is professor of medicine and pathology and head of the Division of Infectious Diseases, University of California, San Diego School of Medicine. Drs. Kirkland and Fierer have worked together for the past 15 years. Currently, they are focusing on the genetic determinants for resistance to infection and on identifying candidates for a coccidioidomycosis vaccine.
Acknowledgment
We are grateful to Dr. Ron Talbot, Director of the Kern County Public Health Laboratory, and Dr. Tom Larwood for sharing unpublished data with us. The experimental work done in our laboratories has been supported by National Institutes for Health grants AI19149 and AI37232 and by the Research Service of the Department of Veterans Affairs.
References
- Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1:7–15. DOIPubMedGoogle Scholar
- Deresinski SC. History of coccidioidomycosis: “dust to dust.” In: Stevens DA, editor. Coccidioidomycosis. New York: Plenum, 1980:1-20.
- Harrison WR, Merbs CF, Leathers CR. Evidence of coccidioidomycosis in the skeleton of an ancient Arizona Indian. J Infect Dis. 1991;164:436–7.PubMedGoogle Scholar
- Cole GT, Sun SH. Arthroconidium-spherule-endospore transformation in Coccidioides immitis. In: Szaniszlo PJ, Harris L, editors. Fungal dimorphism: with emphasis on fungi pathogenic for humans. New York: Plenum, 1985:281-333.
- Pappagianis D. Epidemiology of coccidioidomycosis. Curr Top Med Mycol. 1988;2:199–238.PubMedGoogle Scholar
- Converse JL. Effect of surface active agents on endosporulation of Coccidioides immitis in a chemically defined medium. J Bacteriol. 1957;74:106–7.PubMedGoogle Scholar
- Kamel SM, Wheat LJ, Garten ML, Bartlett MS, Tansey MR, Tewari RP. Production and characterization of murine monoclonal antibodies to Histoplasma capsulatum yeast cell antigens. . Infect Immun. 1989;57:896–901.PubMedGoogle Scholar
- Lacy GH, Swatek FE. Soil ecology of Coccidioides immitis at Amerindian middens in California. Appl Microbiol. 1974;27:379–88.PubMedGoogle Scholar
- Maddy KT. The geographic distribution of Coccidioides immitis and possible ecologic implications. Ariz Med. 1958;15:178–88.PubMedGoogle Scholar
- Smith CE, Beard RR, Rosenberger HG, Whiting EG. Effect of season and dust control on coccidioidomycosis. JAMA. 1946;132:833–8.
- Pappagianis D, Einstein H. Tempest from Tehachapi takes toll or Coccidioides conveyed aloft and afar. West J Med. 1978;129:527–30.PubMedGoogle Scholar
- CDC. Coccidioidomycosis following the Northridge earthquake—California, 1994. MMWR. 1994;43:194–5.PubMedGoogle Scholar
- Johnson WM. Occupational factors in coccidioidomycosis. J Occup Med. 1981;23:367–74. DOIPubMedGoogle Scholar
- Werner SB, Pappagianis D. Coccidioidomycosis in northern California—an outbreak among archeology students near Red Bluff. Calif Med. 1973;119:16–20.PubMedGoogle Scholar
- Smith CE, Beard RR, Whiting EG, Rosenberg HG. Varieties of coccidioidal infection in relation to the epidemiology and control of diseases. Am J Public Health. 1946;36:1394–402. DOIGoogle Scholar
- Smith CE. Epidemiology of acute coccidioidomycosis with erythema nodosum (“San Joaquin” or “Valley Fever”). Am J Public Health. 1940;30:600–11. DOIGoogle Scholar
- Baker EJ, Hawkins JA, Waskow EA. Surgery for coccidioidomycosis in 52 diabetic patients with special reference to related immunologic factors. J Thorac Cardiovasc Surg. 1978;75:680–7.PubMedGoogle Scholar
- Smith CE, Beard RR, Saito MT. Pathogenesis of coccidioidomycosis with special reference to pulmonary cavitation. Ann Intern Med. 1948;29:623–55.PubMedGoogle Scholar
- Williams PL, Sable DL, Mendez P, Smyth LT. Symptomatic coccidioidomycosis following a severe natural dust storm—an outbreak at the Naval Air Station, Lemoore, Calif. Chest. 1979;76:566–70. DOIPubMedGoogle Scholar
- Kirkland TN, Fierer J. Inbred mouse strains differ in resistance to lethal Coccidiodes immitis infection. Infect Immun. 1983;40:912.PubMedGoogle Scholar
- Kirkland TN, Fierer J. Genetic control of resistance to Coccidioides immitis: a single gene that is expressed in spleen cells determines resistance. J Immunol. 1985;135:548–52.PubMedGoogle Scholar
- Cox RA, Kennell W. Suppression of T-lymphocyte response by Coccidioides immitis antigen. Infect Immun. 1988;56:1424–9.PubMedGoogle Scholar
- Wack EE, Ampel NM, Galgiani JN, Bronnimann DA. Coccidioidomycosis during pregnancy—an analysis of ten cases among 47,120 pregnancies. Chest. 1988;94:376–9. DOIPubMedGoogle Scholar
- Powell BL, Drutz DJ, Huppert M, Sun SH. Relationship of progesterone- and estradiol-binding proteins in Coccidioides immitis to coccidioidal dissemination in pregnancy. Infect Immun. 1983;40:478–85.PubMedGoogle Scholar
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. DOIPubMedGoogle Scholar
- Beaman L, Pappagianis D, Benjamini E. Significance of T-cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977;17:580–5.PubMedGoogle Scholar
- Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy. Clin Infect Dis. 1993;16:349–54.PubMedGoogle Scholar
- Jinadu BA. Valley Fever Task Force report on the control of Coccidioides immitis.Bakersfield, CA: Kern Country Health Department, 1995.
- Pappagianis D. Marked increase in cases of coccidioidomycosis in California: 1991, 1992, and 1993. Clin Infect Dis. 1994;19:S14–8.PubMedGoogle Scholar
- Dodge RR, Lebowitz MD, Barbee R, Burrows B. Estimates of C. immitis infection by skin test reactivity in an endemic community. Am J Public Health. 1985;75:863–5. DOIPubMedGoogle Scholar
- Cohen IM, Galgiani JN, Potter D, Ogden DA. Coccidioimomycosis in renal replacement therapy. Arch Intern Med. 1982;142:489–94. DOIPubMedGoogle Scholar
- Rutala PJ, Smith JW. Coccidioidomycosis in potentially compromised hosts: the effect of immunosuppressive therapy in dissemination. Am J Med Sci. 1978;275:283–95. DOIPubMedGoogle Scholar
- Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigenspecific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–52.PubMedGoogle Scholar
- Bronnimann DA, Adam RD, Galgiani JN, Habib MP, Peterson EA, Porter B, Coccidioidomycosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1987;106:372–9.PubMedGoogle Scholar
- Fish DG, Ampel NM, Galgiani JN, Dols CL, Kelly PC, Johnson CH, Coccidioidomycosis during human immunodeficiency virus infection—a review of 77 patients. Medicine (Baltimore). 1990;69:384–91. DOIPubMedGoogle Scholar
- Ampel NM, Dols CL, Galgiani JN. Coccidioidomycosis during human immunodeficiency virus infection: results of a prospective study in a coccidioidal endemic area. Am J Med. 1993;94:235–40. DOIPubMedGoogle Scholar
- Galgiani JN, Catanzaro A, Cloud GA, Higgs J, Friedman BA, Larsen RA, Fluconazole therapy for coccidioidal meningitis: the NIAID-Mycoses Study Group. Ann Intern Med. 1993;119:28–35.PubMedGoogle Scholar
- Tucker RM, Denning DW, Dupont B, Stevens DA. Itraconazole therapy for chronic coccidioidal meningitis. Ann Intern Med. 1990;112:108–12.PubMedGoogle Scholar
- Dewsnup DH, Galgiani JN, Leviner BE, Sharkey-Mathin PK, Fierer J, Stevens DA. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med. 1996;124:305–10.PubMedGoogle Scholar
- Catanzaro A, Fierer J, Friedman PJ. Fluconazole in the treatment of persistent coccidioidomycosis. Chest. 1990;97:666–9. DOIPubMedGoogle Scholar
- Graybill JR, Stevens DA, Galgiani JN, Dismukes WE, Cloud GA, . Itraconazole treatment of coccidioidomycosis. Am J Med. 1990;89:282–90. DOIPubMedGoogle Scholar
- Catanzaro A, Galgiani JN, Levine BE, Sharkey-Mathis PK, Fierer J, Stevens DA, Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. NIAID Mycoses Study Group. Am J Med. 1995;98:249–56. DOIPubMedGoogle Scholar
- Levine HB, Cobb JM, Smith CE. Immunogenicity of spherule-endospore vaccines of Coccidioides immitis for mice. J Immunol. 1961;87:218–27.PubMedGoogle Scholar
- Pappagianis D, Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am Rev Respir Dis. 1993;148:656–60.PubMedGoogle Scholar
- Huppert M, Spratt NS, Vukovich KR, Sun SH, Rice EH. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978;20:541–51.PubMedGoogle Scholar
- Cox RA, Britt LA. Isolation and identification of an exoantigen specific for Coccidioides immitis. Infect Immun. 1986;52:138–43.PubMedGoogle Scholar
- Cole GT, Kirkland TN, Sun SH. An immunoreactive, water-soluble conidial wall fraction of Coccidioides immitis. Infect Immun. 1987;55:657–67.PubMedGoogle Scholar
- Cole GT, Kirkland TN, Zhu M, Yuan L, Sun SH, Hearn VN. Immunoreactivity of a surface wall fraction produced by spherules of Coccidioides immitis. Infect Immun. 1988;56:2695–701.PubMedGoogle Scholar
- Dugger KO, Galgiani JN, Ampel NM, Sun SH, Magee DM, Harrison J, . An immunoreactive apoglycoprotein purified from Coccidioides immitis. . Infect Immun. 1991;59:2245–51.PubMedGoogle Scholar
- Galgiani JN, Sun SH, Dugger KO, Ampel NM, Grace GC, Harrison J, An arthroconidial spherule antigen of Coccidioides immitis: differential expression during in vitro fungal development and evidence for humoral response in humans after infection or vaccination. Infect Immun. 1992;60:2627–35.PubMedGoogle Scholar
- Kirkland TN, Zhu SW, Cruse D, Hsu LL, Seshan KR, Cole GT. Coccidioides immitis fractions which are antigenic for immune T lymphocytes. Infect Immun. 1991;59:3952–61.PubMedGoogle Scholar
- Wycoff E, Pishco J, Kirkland TN, Cole GT. Cloning and expression of a T-cell reactive protein from Coccidioides immitis: homology to 4-hydroxyphenylpyruvate dixygenase and the mammalian Fantigen. Gene. 1995;161:107–11. DOIPubMedGoogle Scholar
- Lecara G, Cox RA, Simpson RB. Coccidioides immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen. Infect Immun. 1983;39:473–5.PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 2, Number 3—July 1996
| EID Search Options |
|---|
|
|
|
|
|
|