Volume 20, Number 11—November 2014
Research
Spread of Streptococcus pneumoniae Serotype 8-ST63 Multidrug-Resistant Recombinant Clone, Spain
Abstract
Since 2004, a total of 131 isolates of Streptococcus pneumoniae multidrug-resistant invasive serotype 8 have been detected in Spain. These isolates showed resistance to erythromycin, clindamycin, tetracycline, and ciprofloxacin. All isolates were obtained from adult patients and shared a common genotype (sequence type [ST]63; penicillin-binding protein 1a [pbp1a], pbp2b, and pbp2x gene profiles; ermB and tetM genes; and a ParC-S79F change). Sixty-eight isolates that required a ciprofloxacin MIC ≥16 μg/mL had additional gyrA gene changes. Serotype 8-ST63 pbp2x sequences were identical with those of antimicrobial drug–susceptible serotype 8-ST53 isolates. Serotype 8-ST63 pbp2b sequences were identical with those of the multidrug-resistant Sweden 15A-ST63 clone. Recombination between the capsular locus and flanking regions of an ST53 isolate (donor) and an ST63 pneumococcus (recipient) generated the novel 15A-ST63 clone. One recombination point was upstream of pbp2x and another was within pbp1a. A serotype 8-ST63 clone was identified as a cause of invasive disease in Spain.
Streptococcus pneumoniae is a frequent cause of community-acquired pneumonia, meningitis, bacteremia, and otitis media in children. The diverse biochemical composition of the capsular polysaccharide results in ≥94 serotypes (1). However, only a few serotypes cause most invasive disease episodes worldwide. Serotype 8 pneumococci cause invasive pneumococcal disease in adults and have been occasionally associated with outbreaks. Nevertheless, isolates of this serotype are rarely found in children as a cause of invasive disease or as colonizers of the nasopharynx (2,3). Few lineages have been identified among serotype 8 pneumococci; the major clone is Netherlands 8-ST53, which has been detected worldwide and is typically susceptible to antimicrobial drugs (4).
The capsular polysaccharide is the major virulence factor of pneumococci and usually determines their ability to act as invasive or colonizing microorganisms (3). In addition to exhibiting the capsule, pneumococci can show low or high genetic diversity, but genotype–serotype association is common. However, this association could be disrupted because of capsular switching caused mainly by recombination of capsular genetic loci. This finding could be a sporadic event, but the recombinant occasionally spreads and could cause pneumococcal disease.
Capsular switching was associated with emergence of a serotype 19A variant of a formerly serotype 4 clone (5) related to immunity pressure sustained after a 7-valent pneumococcal conjugate vaccine was introduced into the United States. This well-known phenomenon was the origin of the major penicillin-resistant clone (serotype 14 variant of the Spain 9V-ST156 clone), which caused invasive pneumococcal disease in Spain in the 2000s (4,6).
Data from the Spanish Reference Laboratory for Pneumococci, which has received pneumococci from Spain since 1979, showed rates of 2.5% to 6.5% for serotype 8 invasive isolates in the last 3 decades. These rates did not show any association with introduction of therapeutic or preventive measures (7,8). Over these decades, serotype 8 pneumococci were usually susceptible to antimicrobial drugs, although some isolates were resistant to erythromycin or tetracycline. Moreover, serotype 8 pneumococci were isolated mainly from adult patients (7,8). However, since 2004, serotype 8 pneumococci have been identified that showed resistance to erythromycin, clindamycin, tetracycline, and ciprofloxacin.
In the present study, we analyzed the evolution and molecular epidemiology of these multidrug-resistant serotype 8 pneumococci. We determined whether the increase in these isolates was associated with dissemination of a new recombinant clone (serotype 8-ST63) capable of causing invasive pneumococcal disease in different areas of Spain.
Bacterial Strains
Isolates received at the Spanish Reference Laboratory for Pneumococci were serotyped by using the quellung reaction and antisera provided by the Staten Serum Institute (Copenhagen, Denmark) (8). During January 2004–December 2012, this laboratory received 22,228 invasive pneumococcal isolates (4,274 from children <15 years of age, 16,506 from persons ≥15 years of age, and 448 from persons for whom age data were not available). Of these isolates, 767 were serotype 8 (3.2%). The proportion of serotype 8 was 4.4% among isolates from adults and 0.4% among isolates from children (8). Of the 767 serotype 8 pneumococci, 131 isolates were resistant to ≥3 antimicrobial drugs (all isolates were from adults). Of these isolates, 119 were available for molecular characterization.
Reference Strains
American Type Culture Collection (ATCC) BAA-661, the reference strain of the Sweden 15A-ST63 clone (resistant to erythromycin, clindamycin, and tetracycline, and decreased susceptibility to penicillin), was used as a control for pulsed-field gel electrophoresis (PFGE) and PCR–restriction fragment length polymorphism (PCR-RLFP) analysis of penicillin-binding protein (PBP) genes. Strains CSUB8370, CSUB8757, and CSUB5364 (all 3 strains are serotype 8, susceptible to antimicrobial drugs, and ST53) were also used as controls for PFGE and PCR-RFLP of PBPs (4,9,10).
Antimicrobial Drug Susceptibility Testing
MICs of antimicrobial drugs for drug-resistant isolates were determined by using the microdilution method, 2%–5% lysed horse blood, and commercially available panels (STRHAE1; Sensititre, East Grunstead, UK) and following the recommendations of the Clinical Laboratory Standards Institute (11,12). S. pneumoniae ATCC 49619 was used for quality control testing. When ciprofloxacin or levofloxacin MICs were >2 μg/mL, these MICs were tested by using the E test (AB Biodisk, Solna, Sweden).
Molecular Typing
Genomic DNA was embedded in agarose plugs and digested with SmaI. Fragments were separated by PFGE in a CHEF-DRIII apparatus (Bio-Rad, Hercules, CA, USA as described (9). PFGE patterns were compared visually with patterns of reference and control strains. Seventeen strains selected from different areas and years were studied by using multilocus sequencing typing (MLST) as described (13). Allele numbers and sequence types (STs) were assigned by using the pneumococcal MLST website (http://www.mlst.net).
PBP Fingerprinting
The analysis of pbp1a, pbp2b, and pbp2x genes was performed by using PCR-RFLP. We used primers described by du Plessis et al. (14) for pbp1a and those described by Gherardi et al. (15) for pbp2b and pbp2x. PCR products were digested with HinfI, and digestion patterns were visually compared. In addition, both strands of PCR PBP gene products of 3 selected strains were purified and sequenced by using amplification primers for pbp2b and pbp2x. For pbp1a amplification, primers and PBP1ASq1 (5′-TAAGGTCTACATGTCTAAT-3′) were used for sequencing purposes.
Resistance Phenotype Characterization
The presence of macrolide resistance genes (ermB, mefA/E) and the tetracycline resistance gene (tetM) was tested by PCR described elsewhere (16). To characterize quinolone resistance for all strains, we used a PCR-RFLP method that detects point mutations at the main quinolone resistance determinant region (QRDR) positions (17,18). In brief, after PCR amplification, parC amplicons were digested with HinfI or SfaNI to detect changes in S79 or D83, respectively. PCR products of parE were digested with HinfI to detect changes in D435. Finally, gyrA amplicons were digested with HinfI (S81) or MboII (E85) (17,18). Control strains with known QRDR mutations were used in each run (19).
PCR Amplification and DNA Sequencing and Analysis
QRDRs sequences were determined for 43 strains (15 with low-level ciprofloxacin resistance [LLCipR] [MIC ≤8 μg/mL] and 28 with high-level ciprofloxacin resistance [HLCipR] [MIC ≥16 μg/mL)]. In brief, gyrA, parC, parE, and gyrB genes were amplified by using PCR and primers and conditions previously described (19). PCR products were purified, and both strands were sequenced by using primers for PCR amplification (19).
Analysis of Recombination Site Upstream of pbp2x
We used sequence alignments of ≈20 kb located upstream of the pbp2x gene of 2 serotype 8 (ST53) strains (2071247 [GenBank accession no. ALBK01000004); and 2081685 [ALBN01000001]), 1 serotype 15A (ST63) strain (GA47179 [AIKX01000002]), and strain G54 (serotype 19F, ST63, CP001015) (20) pneumococcal genomes to design 7 sets of PCR primers. These primers were used to identify the putative recombination event upstream of pbp2x (Table).
Description and Spread of Multidrug-Resistant Strain
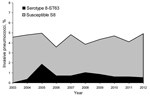
During 2003–2012, among invasive strains collected from adults, the prevalence of serotype 8 ranged between 3.6% and 5.0% (Figure 1). In 2004, four invasive multidrug-resistant serotype 8 isolates were detected at the Spanish Reference Laboratory from 3 hospitals in Madrid (central Spain). Multidrug-resistant serotype 8 isolates were also detected in eastern (2005), southern (2006), and northwestern (2007) Spain. During 2009−2012, multidrug-resistant serotype 8 isolates were detected among invasive isolates from 7 autonomous communities. In the Madrid area, serotype 8-ST63 isolates were detected mainly in adult HIV-infected patients (21). All multidrug-resistant serotype 8 isolates were from adults (78.7% from male patients). Sources of isolation of multidrug-resistant serotype 8 pneumococci were blood (n = 116), pleural fluid (n = 6), cerebrospinal fluid (n = 2), fluid from eyes with endophthalmitis (n = 6), and abscess fluid (n = 1).
All 131 multidrug-resistant serotype 8 pneumococci were susceptible to penicillin (MIC <0.03 µg/mL), cefotaxime (MIC <0.03 μg/mL), and amoxicillin (MIC <0.06 μg/mL) and resistant to erythromycin (MIC >128 μg/mL), clindamycin (MIC >128 μg/mL), and tetracycline (MIC >64 μg/mL). Ciprofloxacin MICs ranged from 2 μg/mL to 64 μg/mL, and 68 strains showed HLCipR (MIC ≥16 μg/mL).
Molecular Typing
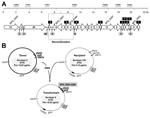
A common PFGE pattern (nearly identical to that of the reference Sweden 15A-ST63 clone) was found among all 119 available serotype 8 isolates with multidrug resistance, which suggested a capsular switching event. This PFGE pattern was different from those of other antimicrobial drug–susceptible serotype 8-ST53 isolates (related to the Netherlands 8-ST53 clone) (Figure 2, panel A). These PFGE patterns were confirmed after MLST characterization of 17 selected multidrug-resistant serotype 8 isolates (all were ST63).
PBP Typing and Analysis
Results of PCR-RFLP analysis of pbp1A, pbp2b, and pbp2x genes of serotype 8-ST63 and antimicrobial drug–susceptible serotype 8-ST53 and of the multidrug-resistant Sweden 15A-ST63 reference strain (ATCC BAA-661) are shown in Figure 2, panel B. Amino acid sequence variation of the PBP1A, PBP2B, and PBP2X proteins of the epidemic strain and 2 control strains is shown in Figure 2, panel C. All serotype 8-ST63 isolates showed the same restriction profile for each pbp gene analyzed, which suggested clonal homogeneity. The restriction profile of pbp2b of the new strain was identical with that of the Sweden 15A-ST63 clone and different from those of serotype 8-ST53 drug-susceptible strains according to PCR-RFLP results. These results were confirmed after sequencing the pbp2b gene amplicons of 3 serotype 8-ST63 isolates, the Sweden 15A-ST63 reference strain, and 2 serotype 8-ST53 isolates (Figure 2, panel C).
The PCR-RFLP profile of pbp2x of serotype 8-ST63 isolates was identical with those of serotype 8-ST53 isolates (related to the Netherlands 8-ST53 clone) and different from that of the Sweden 15A-ST63 reference strain. These results were confirmed after sequencing the pbp2b gene amplicons of 3 serotype 8-ST63 isolates, the Sweden 15A-ST63 reference strain, and the serotype 8-ST53 isolates.
All serotype 8-ST63 isolates had the same PCR-RFLP profile for pbp1a. This profile was different from those of antimicrobial drug–susceptible serotype 8-ST53 and nearly identical with the profile of Sweden 15A-ST63. Nucleotide sequence variation in the pbp1a gene of serotype 8-ST53, serotype 8-ST63, and Sweden 15A-ST63, determined by using S. pneumoniae R6 as a reference, is shown in Figure 3. Using R6 nomenclature for the pbp1a gene, we found that sequences of serotype 8-ST63 and Sweden 15A-ST63 were identical for nt 1–1341. Sequences of serotype 8-ST53, serotype 8-ST63, and Sweden 15A-ST63 were identical at nt 1342–1353. Sequences of pbp1a for nt 1354–1970 of serotype 8-ST63 were identical with those of antimicrobial drug–susceptible serotype 8-ST53. These results suggest that a recombination point was located in the 3′ end of the pbp1a gene.
Recombination Event Upstream of pbp2x
To determine the position of the recombination event that gave rise to the serotype 8-ST63 strain, we searched the public databases for genomic sequences located upstream of the pbp2x gene corresponding to serotype 8-ST53 and serotype 15A-ST63 pneumococcal strains. Draft genomic sequences of 2 serotype 8-ST53 strains (2071247 [ALBK01000004]) and 2081685 [ALBN01000001]) were found. These sequences differed only at 5 positions in an ≈20-kb region. The draft sequence of the genome of a single serotype 15A-ST63 (strain GA47179 [ AIKX01000002]) was also used. Moreover, when we compared this sequence (serotype 15A-ST63) with complete genomes of S. pneumoniae strains, we found an excellent match with that of strain G54 (serotype 19F; ST63); only 2 nt changes occurred in a 20,792-bp overlap.
The G54 strain has been shown to be a type 19F transformant of a serogroup 15 isolate (20). Nucleotide alignment of the 4 strains mentioned above showed several polymorphic sites, which enabled clear (although somehow limited) discrimination between ST53 and ST63 strains (Figure 4, panel A). Different fragments of the ≈20-kb region located upstream of pbpx2 were amplified by PCR and sequenced (Table; Figure 4, panel A). Sequence comparisons showed that the recombination event between the donor DNA of a serotype 8 (ST53) strain and a recipient strain of serotype 15A (ST63) had occurred in a ≈3.8-kb region that contained genes SPG_0286 and SPG_0290 (using the strain G54 notation). Further attempts to determine more precisely the location of the recombination event were not made because this region has only 17 polymorphic sites. A scheme of the possible recombination event in which capsular loci and flanking regions (pbp2x and pbp1a) of a serotype 8-ST53 strain were acquired by ST63 pneumococci is shown in Figure 4, panel B.
Molecular Characterization of Drug Resistance
The ermB gene (which confers the macrolide–lincosamide–streptogramin B phenotype) and the tetM gene (which confers tetracycline resistance) were detected by PCR. Both genes are usually present in pneumococci related to the Sweden 15A-ST63 clone (16). These genes are located in the composite element Tn5251 (Figure 4, panel B) (20).
Among 131 serotype 8-ST63 strains, 63 showed LLCipR (MICs 2 μg/mL–8 μg/mL) and 68 showed (HLCipR (MICs ≥16 μg/mL). An increase in the proportion of serotype 8-ST63 isolates with HLCipR was observed: from 19.5% (8/41) during 2004−2006 to 86.7% (26/30) during 2010−2012 (p<0.001). PCR-RFLP analysis showed that all 119 available serotype 8-ST63 isolates that were studied lacked the restriction site for HinfI in parC, which is associated with a change in S79. This change was confirmed to be an S79F after sequencing 43 isolates (15 LLCipR and 28 HLCipR). Fifty-nine of 61 available LLCipR isolates (all with the S79 change) had levofloxacin MICs ≤2 μg/mL (susceptible).
A total of 58 of 68 HLCipR isolates were available for analysis. Of these 58 isolates, 47 lacked the HinfI restriction site in gyrA, which is associated with a mutation at S81; 10 isolates lacked the MboII restriction site related to a gyrA change at E85; and 1 isolate lacked the HinfI and MboII restriction sites that were assumed to be caused by changes in S81 and E85 in gyrA. The results of gyrA QRDR sequencing of 28 selected HLCipR isolates showed various gyrA changes (S81F [n = 23]; S81Y [n = 2]; E85K [n = 3]; and S81F and E85K [n = 1 each]). No parE changes were detected by PCR-RFLP or sequencing. Serotype 8-ST63 isolates for which QRDRs were sequenced showed polymorphisms for parC (G77[GGA]), parE (I460V), and gyrA (Y75[TAT]) that were identical with those of the Sweden 15A-ST63 reference strain and other pneumococci of this lineage (21,22).
In this study, we described the emergence and spread of a pneumococcal clone that originated by recombination of a ciprofloxacin-resistant and multidrug-resistant strain related to the Sweden 15A-ST63 clone, which acted as a recipient of DNA, and a serotype 8 strain with ST53, which acted as a donor of DNA. The new recombinant clone (serotype 8-ST63) was initially confined to the metropolitan area of Madrid and was mainly associated with HIV-infected patients (21). However, in the past 5 years, the new clone has been detected in 9 other regions in Spain, which indicates an ability to spread. Two characteristics merged in this new clone, the invasive disease potential of serotype 8 and the antimicrobial drug resistance of ST63, which suggested that the recombinant could be a new antimicrobial drug–resistant clone.
The capsular polysaccharide is the major factor in determining the invasive disease potential of a given isolate. Serotype 8 has been associated with high invasive disease potential when a large collection of invasive and colonizing isolates were compared (23). Moreover, capsular serotype 8 was associated with high attack rates; 30 cases of invasive pneumococcal disease/100,000 carriage acquisitions were detected (2). Conversely, serotype 15A pneumococci show low invasive potential and are usually associated with ST63 and resistance to macrolides, clindamycin (ermB) and tetracycline (tetM) (2,16). The overall frequency of clonal complex (CC) 63 among invasive isolates from adults was low: 2.5% (22/899) in 1997–2008 (4). Moreover, in 3 studies conducted in Spain with quinolone-resistant and macrolide-resistant pneumococci, CC63 was 1 of the most prevalent clones (16,19,22).
The results of the present study suggest that the new strain could be the result of a recombination event between a serotype 15A-ST63 pneumococcal isolate that contained the S79F change in ParC and a serotype 8-ST53 pneumococcus. In this event, the recombination fragment included the pbp2x gene and part of the pbp1a gene. The recombination of the cps locus and flanking regions has been observed by other authors, (6,24,25), which suggests that it is a common biologic process in the evolution of pneumococci.
The new strain, serotype 8-ST63, is penicillin susceptible (MIC <0.01 μg/mL), and ST63 isolates with other serotypes (15F, 15A, 15B, 19F, and 19A) are usually penicillin resistant (MIC range 0.12 μg/mL−0.5 μg/mL) (10,16,22). The penicillin resistance of CC63 isolates is associated with a Q552E change in PBP2X (26). The acquisition of pbp2x genes from serotype 8-ST53, without changes involved in β-lactam resistance, by the recombinant strain explains its penicillin susceptibility.
There have been other examples of recombinant clones caused by capsular switching that successfully spread. For instance, in the past decade, acquisition of serotype 14 by pneumococci of serotype 9V of ST156 has been associated with the worldwide increase of serotype 14 as a cause of invasive pneumococcal disease (4,6). Moreover, exchange of capsular genes with pbp genes has been documented in the serotype 19A-ST320 clone that spread worldwide and is a major cause of multidrug-resistant invasive and noninvasive disease (27,28). In the United States, recombination of capsular and pbp genes has been observed as a vaccine escape of a penicillin-susceptible serotype 4 that acquired the capsular genes and PBPs of a serotype 19A strain. This recombinant strain had a nonvaccine serotype and an antimicrobial drug resistance pattern that favored its spread in the United States. (5). To the best of our knowledge, the new recombinant clone (serotype 8-ST63) described here has not been detected outside Spain.
A report from South Africa described the spread of 2 quinolone-resistant strains that caused invasive disease and colonized children; most of these children were receiving treatment with antimicrobial drugs (including levofloxacin) for multidrug-resistant tuberculosis (29). Moreover, in previous studies, our group and others have observed some clustered infections, with a limited number of cases caused by quinolone-resistant pneumococci in a specific geographic area (19,30). The recombinant strain (serotype 8-ST63) described in the present study has been detected in several cities in Spain, which demonstrates its ability to disseminate.
Most isolates of this new clone with ciprofloxacin resistance required MICs of levofloxacin that were in the susceptible range according to recommendations of the Clinical and Laboratory Standards Institute (12). Because these strains had a first-step mutation, use of fluoroquinolones to treat infections could become a problem. At least 1 death, resulting from therapeutic failure of levofloxacin, has been documented: the patient had pneumococcal pneumonia caused by a serotype 8-ST63 pneumococci in Seville, Spain. In this case, the initial strain had the S79F change in ParC and required a levofloxacin MIC of 1 μg/mL. After levofloxacin therapy, high-level drug resistance developed in this strain after a second change in GyrA (31).
In the 1990s, use of antimicrobial drugs was high in Spain. During this period, penicillin-resistant and multidrug-resistant clones spread over the country, caused invasive disease, and colonized healthy children. Since that time, use of antimicrobial drugs has decreased in Spain (32). Together with vaccination with the 7-valent pneumococcal conjugate vaccine, the decrease in use of β-lactams probably contributed to the recent decrease in penicillin-resistance and multidrug-resistance rates (4,8). In contrast, data from the Spanish Medicines Agency (http//agemed.es) indicate that levofloxacin consumption has increased from 0.2 defined daily doses/1,000 persons/day (DDD) in 2002 to 0.4 DDD in 2006 and 0.6 DDD in 2012. This antimicrobial drug pressure could have favored spread of the fluoroquinolone-resistant clone in Spain and resulted in the increase in the proportion of HLCipR among serotype 8-ST63 isolates.
Children are the main reservoir for pneumococci, but quinolones are seldom prescribed for this population. Thus, isolation of quinolone-resistant pneumococci from children is rare. We have not detected any serotype 8-ST63 pneumococci in isolates from children. However, in a plausible scenario, the recombinant clone could colonize the nasopharynx of children and become a major source for dissemination.
In conclusion, emergence and spread of the serotype 8-ST63 clone that originated by genetic interchange of capsular genes and their flanking regions, including pbp1a and pbp2x genes, have been detected in Spain. The 2 clones involved in capsular switching were Netherlands 8-ST53 and Sweden 15A-ST63. Surveillance is needed to clarify the dynamics of this new multidrug-resistant clone as cause of pneumococcal disease.
Dr Ardanuy is a clinical microbiologist and researcher at the Microbiology Department, Hospital Univesitari de Bellvitge, L’Hospitalet de Llobregat, Barcelona, Spain. Her research interests are molecular epidemiology and antimicrobial drug resistance in bacteria.
Acknowledgments
We thank Imperial College, London, for use of the S. pneumoniae MLST website (supported by the Wellcome Trust), and Montserrat Alegre and Meritxell Cubero for excellent technical support.
This study was supported by a grant from Fondo de Investigaciones Sanitarias de la Seguridad Social (PI11/00763), Plan Nacional de I + D + I of the Ministerio de Ciencia e Innovación of Spain (BIO2011-25343), and Centros de Investigación Biomédica en Red (CIBER) de Enfermedades Respiratorias (CIBERES-CB06/06/0037), an initiative of the Instituto de Salud Carlos III, Madrid, Spain. C.A., A.F., and J.L. received support from Pfizer for a project independent of the present study.
References
- Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J, Abeygunwardana C, Biochemical, genetic and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem. 2012;287:27885–94. DOIPubMedGoogle Scholar
- Sleeman KL, Griffiths D, Shackley F, Diggle L, Gupta S, Maiden MC, Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis. 2006;194:682–8. DOIPubMedGoogle Scholar
- Sjöström K, Spindler C, Ortqvist A, Kalin M, Sandgren A, Kühlmann-Berenzon S, Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis. 2006;42:451–9. DOIPubMedGoogle Scholar
- Ardanuy C, Tubau F, Pallares R, Calatayud L, Domínguez MA, Rolo D, Epidemiology of invasive pneumococcal disease among adult patients in Barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997–2007. Clin Infect Dis. 2009;48:57–64. DOIPubMedGoogle Scholar
- Brueggemann AB, Pai R, Crook DW, Beall B. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 2007;3:e1628–36.
- Coffey TJ, Daniels M, Enright MC, Spratt BG. Serotype 14 variants of the Spanish penicillin-resistant serotype 9V clone of Streptococcus pneumoniae arose by large recombinational replacements of the cpsA-pbp1a region. Microbiology. 1999;145:2023–31. DOIPubMedGoogle Scholar
- Fenoll A, Jado I, Vicioso D, Pérez A, Casal J. Evolution of Streptococcus pneumoniae: serotypes and antibiotic resistance in Spain. Update (1990 to 1996). J Clin Microbiol. 1998;36:3447–54 .PubMedGoogle Scholar
- Fenoll A, Granizo JL, Aguilar L, Giménez MJ, Aragoneses-Fenoll L, Hanquet G, Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J Clin Microbiol. 2009;47:1012–20. DOIPubMedGoogle Scholar
- McGee L, McDougal L, Zhou J, Spratt BG, Tenover FC, George R, Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J Clin Microbiol. 2001;39:2565–71. DOIPubMedGoogle Scholar
- Sá-Leão R, Tomasz A, de Lencastre H. Multilocus sequence typing of Streptococcus pneumoniae clones with unusual drug resistance patterns: genetic backgrounds and relatedness to other epidemic clones. J Infect Dis. 2001;184:1206–10. DOIPubMedGoogle Scholar
- Clinical and Laboratory Standard Institute. Methods for dilution antimicrobial susceptibility test for bacteria that growth aerobically. 8th ed. Vol. 29. Approved standard. CLSI/NCCLS document M7–A8. Wayne (PA): The Institute; 2009.
- Clinical and Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing; Twenty-first informational supplement. Vol. 31. CLSI/NCCLS document M100–S21. Wayne (PA): The Institute; 2011.
- Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–60. DOIPubMedGoogle Scholar
- du Plessis M, Bingen E, Klugman KP. Analysis of penicillin-binding protein genes of clinical isolates of Streptococcus pneumoniae with reduced susceptibility to amoxicillin. Antimicrob Agents Chemother. 2002;46:2349–57. DOIPubMedGoogle Scholar
- Gherardi G, Whitney CG, Facklam RR, Beall B. Major related sets of antibiotic-resistant pneumococci in the United States as determined by pulsed-field gel electrophoresis and pbp1a-pbp2b-pbp2x-dhf restriction profiles. J Infect Dis. 2000;181:216–29. DOIPubMedGoogle Scholar
- Calatayud L, Ardanuy C, Tubau F, Rolo D, Grau I, Pallarés R, Serotype and genotype replacement among macrolide-resistant invasive pneumococci in adults: mechanisms of resistance and association with different transposons. J Clin Microbiol. 2010;48:1310–6. DOIPubMedGoogle Scholar
- Alonso R, Galimand M, Courvalin P. An extended PCR-RFLP assay for detection of parC, parE and gyrA mutations in fluoroquinolone-resistant Streptococcus pneumoniae. J Antimicrob Chemother. 2004;53:682–3. DOIPubMedGoogle Scholar
- Pan XS, Ambler J, Mehtar S, Fisher LM. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–6 .PubMedGoogle Scholar
- de la Campa AG, Balsalobre L, Ardanuy C, Fenoll A, Pérez-Trallero E, Liñares J. Spanish Pneumococcal Infection Study Network G03/103. Fluoroquinolone resistance in penicillin-resistant Streptococcus pneumoniae clones, Spain. Emerg Infect Dis. 2004;10:1751–9. DOIPubMedGoogle Scholar
- Dopazo J, Mendoza A, Herrero J, Caldara F, Humbert Y, Friedli L, Annotated draft genomic sequence from a Streptococcus pneumoniae type 19F clinical isolate. Microb Drug Resist. 2001;7:99–125. DOIPubMedGoogle Scholar
- Sanz JC, Cercenado E, Marín M, Ramos B, Ardanuy C, Rodríguez-Avial I, Multidrug-resistant pneumococci (serotype 8) causing invasive disease in HIV+ patients. Clin Microbiol Infect. 2011;17:1094–8. DOIPubMedGoogle Scholar
- de la Campa AG, Ardanuy C, Balsalobre L, Pérez-Trallero E, Marimón JM, Fenoll A, Changes in fluoroquinolone-resistant Streptococcus pneumoniae after 7-valent conjugate vaccination, Spain. Emerg Infect Dis. 2009;15:905–11. DOIPubMedGoogle Scholar
- Sá-Leão R, Pinto F, Aguiar S, Nunes S, Carriço JA, Frazão N, Analysis of invasiveness of pneumococcal serotypes and clones circulating in Portugal before widespread use of conjugate vaccines reveals heterogeneous behavior of clones expressing the same serotype. J Clin Microbiol. 2011;49:1369–75. DOIPubMedGoogle Scholar
- Coffey TJ, Dowson CG, Daniels M, Zhou J, Martin C, Spratt BG, Horizontal transfer of multiple penicillin-binding protein genes, and capsular biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2255–60. DOIPubMedGoogle Scholar
- Wyres KL, Lambertsen LM, Croucher NJ, McGee L, von Gottberg A, Liñares J, Pneumococcal capsular switching: a historical perspective. J Infect Dis. 2013;207:439–49. DOIPubMedGoogle Scholar
- Pernot L, Chesnel L, Le Gouellec A, Croize J, Vernet T, Dideberg O, A PBP2X from a clinical isolate of Streptococcus pneumoniae exhibits an alternative mechanism for reduction of susceptibility to β-lactam antibiotics. J Biol Chem. 2004;279:16463–70. DOIPubMedGoogle Scholar
- Moore MR, Gertz RE Jr, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016–27. DOIPubMedGoogle Scholar
- Ardanuy C, Rolo D, Fenoll A, Tarragó D, Calatayud L, Liñares J. Emergence of a multidrug-resistant clone (ST320) among invasive serotype 19A pneumococci in Spain. J Antimicrob Chemother. 2009;64:507–10. DOIPubMedGoogle Scholar
- Wolter N, du Plessis M, von Gottberg A, de Gouveia L, Klugman KP. Molecular characterization of emerging non-levofloxacin-susceptible pneumococci isolated from children in South Africa. J Clin Microbiol. 2009;47:1319–24. DOIPubMedGoogle Scholar
- Ho P-L, Que T-L, Chiu SS, Yung RWH, Ng T-K, Tsang DNC, Fluoroquinolone and other antimicrobial resistance in invasive pneumococci, Hong Kong, 1995−2001. Emerg Infect Dis. 2004;10:1250–7. DOIPubMedGoogle Scholar
- de Cueto M, Rodríguez JM, Soriano MJ, López-Cerero L, Venero J, Pascual A. Fatal levofloxacin failure in treatment of a bacteremic patient infected with Streptococcus pneumoniae with a preexisting parC mutation. J Clin Microbiol. 2008;46:1558–60. DOIPubMedGoogle Scholar
- Campos J, Ferech M, Lázaro E, de Abajo F, Oteo J, Stephens P, Surveillance of outpatient antibiotic consumption in Spain according to sales data and reimbursement data. J Antimicrob Chemother. 2007;60:698–701. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 20, Number 11—November 2014
| EID Search Options |
|---|
|
|
|
|
|
|
![Thumbnail of A) Pulsed-field gel electrophoresis patterns of chromosomal DNA of Streptococcus pneumoniae isolates after digestion with SmaI. Lane 1, Sweden 15A-ST63 (American Type Culture Collection [ATCC] BAA-661); lanes 2−5, serotype 8-ST63; lanes 6 and 7, serotype 8-ST53; lane M, molecular mass markers. B) PCR–restriction fragment length polymorphism patterns of penicillin-binding protein genes pbp1A, pbp2b, and pbp2x. Lane 1, Sweden 15A-ST63 (ATCC BAA-661) lanes 2−4: serotype 8-ST63; lanes 5](/eid/images/13-1215-F2-tn.jpg)

Please use the form below to submit correspondence to the authors or contact them at the following address:
Carmen Ardanuy, Servicio de Microbiología, Hospital Universitari de Bellvitge, Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain
Top