Volume 21, Number 12—December 2015
Letter
Severe Ocular Cowpox in a Human, Finland
To the Editor: We describe cowpox with corneal involvement in a 31-year-old atopic woman who lived in southern Finland and was unvaccinated for smallpox. In August 2009, she noticed irritation and edema in her right eye and sought care from a local physician; she started topical antimicrobial drug therapy and oral cephalexin 2 days later. Over the following week, fever developed (37.6°C –39.0°C), edema developed on half her face, the eye became increasingly painful, and visual acuity decreased. The conjunctiva was severely chemotic and hyperemic, but the cornea was clear and the other eye unaffected.
Microbiologic samples taken from the eye 11 days after onset showed neither bacteria nor respiratory viruses. Orbital tomography results were normal. The patient was hospitalized, and broad-spectrum intravenous antimicrobial treatment (meropenem, vancomycin, valacyclovir, and fluconazole) was started, combined with topical corticosteroids and antimicrobial drugs. Within 2 weeks, the conjunctiva showed necrosis, and epithelial erosions appeared in the lower cornea, but visual acuity normalized (Technical Appendix Figure, panels A, B).
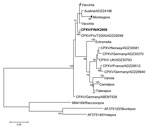
A strong cytopathic effect was observed in Vero cells infected with conjunctival swab (Technical Appendix Table 1), but the virus was unidentifiable by routine methods. In electron microscopy, cell culture and tear fluid samples contained particles with typical orthopoxvirus (OPV) morphology. PCRs for hemagglutinin (1) and 14-kDa genes (2) verified OPV infection. Additional PCRs and sequencing confirmed zoonotic cowpox virus (CPXV) with strain designation FIN/K2009. Nucleotide sequences of the hemagglutinin, thymidine kinase, and A-type inclusion body protein genes were identical to those of CPXV strains T2000 and E1989 previously identified in Finland (3). In phylogenetic analysis (Figure), CPXV/FIN/K2009 clustered with strains from Austria and shared ancestry with vaccinia virus. OPV IgG and IgM were detected by immunofluorescence assay (3) in serum samples up to 5 months after symptom onset (Technical Appendix Table 1).
The patient was started on intravenous polyclonal gammaglobulin and topical trifluorothymidine with in vitro anti-OPV effects; nevertheless, corneal erosions enlarged, corneal stromal edema ensued, and intraocular pressure increased (online Technical Appendix Figure, panel C), suggesting trabeculitis. Topical autologous serum drops had no effect. Periorbital edema slowly resolved, but corneal erosions persisted. Amniotic membrane transplantation (AMT) (4) was performed 2.5 months after onset. The inferior cornea melted, and the cornea lost transparency (online Technical Appendix Figure, panel D). AMT was repeated twice at 1-month intervals because of corneal thinning.
At 3.5 months after symptom onset, tecovirimat (400 mg 2×/d) was given orally for 14 days. Despite treatment, ocular OPV PCR test results remained positive until 9 months after onset (Technical Appendix Table 1), and corneal melting progressed (Technical Appendix Figure, panel E). Corneal collagen cross-linking and a fourth AMT were performed at 5 months after onset with partial success (Technical Appendix Figure, panel F).
At 1 year after symptom onset, corneal limbal stem cell deficiency with deep corneal neovascularization was evident. Autologous limbal stem cell transplantation from the patient’s other eye and another AMT were performed, resulting in stable corneal surface 2 months later (Technical Appendix Figure, panel G). Neovascularization regressed, the cornea cleared, and vision improved (Technical Appendix Figure, panels H, I).
Cowpox is transmitted to humans sporadically from rodents or cats (5). We snap-trapped 23 wild rodents from the yard of the patient’s home and from an adjacent meadow and trapped 136 rodents from 3 other regions 30–100 km from the patient’s home (Technical Appendix Table 2). We also collected 8 environmental samples from the patient’s storehouse. In accordance with the Finnish Act on Use of Animals for Experimental Purposes (62/2006) and the Finnish Animal Experiment Board’s later decision (May 16, 2007), the animal capture technique used is not an animal experiment and requires no ethics license.
Diluted blood for IFA was collected from all rodents (6), and DNA was extracted from rodent liver and lungs and from environmental samples. One vole and 1 mouse from the meadow were seropositive for OPV; however, no CPXV DNA was amplifiable in the samples from the liver, lungs, or environment (online Technical Appendix Table 2).
CPXV infection may manifest in severe ocular forms along with self-limiting cutaneous pocks (5). Our patient had keratitis with no other identifiable cause but CPXV. Culture and PCR from early conjunctival samples and serology confirmed the etiologic diagnosis.
Our case and that of another report (7) highlight the challenges of treating cowpox keratitis. Topical and systemic antiviral drugs and AMT appear ineffective during the acute phase. Corneal melting and scarring continued as long as CPXV was observed and until combined limbal stem cell and AMT treatment had favorable outcomes. Anamnesis of therapy-resistant keratitis should include information on rodent contacts.
We dated the infection to mid-August (incubation 7–21 days). Catching OPV-IgG–positive rodents close to the patient’s home 2 months after onset showed that OPVs were circulating in the local rodent population and indicated the putative role of CPXV-infected voles as the source of infection.
The latest cowpox outbreak in Central Europe involved several humans and pets (8). This patient was born in 1977, after Finland ceased smallpox vaccinations. Declining cross-reactive smallpox-vaccination immunity enables emergence of unusual cowpox infections in humans (9).
Acknowledgments
We thank Katja Koskela with the Finnish Defence Forces for help with the sequence analysis program.
This research was partially funded by EU grant GOCE-2003-010284 EDEN.
References
- Putkuri N, Piiparinen H, Vaheri A, Vapalahti O. Detection of human orthopoxvirus infections and differentiation of smallpox virus with real-time PCR. J Med Virol. 2009;81:146–52. DOIPubMedGoogle Scholar
- Olson VA, Laue T, Laker MT, Babkin IV, Drosten C, Shchelkunov SN, Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J Clin Microbiol. 2004;42:1940–6. DOIPubMedGoogle Scholar
- Pelkonen PM, Tarvainen K, Hynninen A, Kallio ERK, Henttonen H, Palva A, Cowpox with severe generalized eruption, Finland. Emerg Infect Dis. 2003;9:1458–61. DOIPubMedGoogle Scholar
- Mattila JS, Korsbäck A, Krootila K, Holopainen JM. Treatment of Pseudomonas aeruginosa keratitis with combined corneal cross-linking and human amniotic membrane transplantation. Acta Ophthalmol. 2013;91:e410–1. DOIPubMedGoogle Scholar
- Haller SL, Peng C, McFadden G, Rothenburg S. Poxviruses and the evolution of host range and virulence. Infect Genet Evol. 2014;21:15–40. DOIPubMedGoogle Scholar
- Kinnunen PM, Henttonen H, Hoffmann B, Kallio ERK, Korthase C, Laakkonen J, Orthopoxvirus infections in Eurasian wild rodents. Vector Borne Zoonotic Dis. 2011;11:1133–40. DOIPubMedGoogle Scholar
- Graef S, Kurth A, Auw-Haedrich C, Plange N, Kern WV, Nitsche A, Clinicopathological findings in persistent corneal cowpox infection. JAMA Ophthalmol. 2013;131:1089–91. DOIPubMedGoogle Scholar
- Becker C, Kurth A, Hessler F, Kramp H, Gokel M, Hoffmann R, Cowpox virus infection in pet rat owners: not always immediately recognized. Dtsch Arztebl Int. 2009;106:329–34.PubMedGoogle Scholar
- Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107:16262–7. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 21, Number 12—December 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Paula M. Kinnunen, Finnish Food Safety Authority Evira, Mustialankatu 3, 00790 Helsinki, Finland
Top