Volume 21, Number 5—May 2015
Dispatch
Culex torrentium Mosquito Role as Major Enzootic Vector Defined by Rate of Sindbis Virus Infection, Sweden, 2009
Abstract
We isolated Sindbis virus (SINV) from the enzootic mosquito vectors Culex torrentium, Cx. pipiens, and Culiseta morsitans collected in an area of Sweden where SINV disease is endemic. The infection rate in Cx. torrentium mosquitoes was exceptionally high (36 infections/1,000 mosquitoes), defining Cx. torrentium as the main enzootic vector of SINV in Scandinavia.
In Sweden, Finland, Russia, and South Africa, Sindbis virus (SINV; family Togaviridae, genus Alphavirus) is an etiologic agent for outbreaks of rash and long-lasting polyarthritis (1). Ecologically, SINV is a zoonotic mosquitoborne virus that naturally circulates in bird populations but only incidentally infects humans (1). Previous detections and isolations of SINV from field-collected mosquitoes identified the ornithophilic mosquitoes Culex pipiens/Cx. torrentium and Culiseta morsitans as possible enzootic vectors of SINV and the generalist mosquitoes Aedes cinereus and Ae. rossicus, which feed on birds and humans, as potential bridge vectors for transmission of the virus from viremic birds to humans (2,3; J.C. Hesson, J.O. Lundström, unpub. data). However, female Cx. torrentium and Cx. pipiens mosquitoes are morphologically indistinguishable, so all previous virus isolates from these species were from pools that may have contained both species. The distinction between Cx. torrentium and Cx. pipiens is necessary because vector competence experiments show great differences between the capacities of the 2 species to become infected with and to transmit SINV (4,5). Cx. torrentium is highly superior to Cx. pipiens as a vector of SINV in the laboratory (4,5), but the extent to which the 2 species are infected in nature is unclear.
We determined the natural SINV infection rates (IRs) in Culex mosquitoes, which were identified by using a newly developed molecular method for reliable identification of Cx. torrentium and Cx. pipiens mosquitoes (6). We also studied the simultaneous occurrence of SINV in Cs. morsitans mosquitoes.
Every 2 weeks during July 13–September 13, 2009, we collected adult female mosquitoes by using 35 miniature light traps from the US Centers for Disease Control and Prevention (Atlanta, GA, USA) baited with carbon dioxide; the traps were set within the regular mosquito surveillance area of the River Dalälven floodplains in central Sweden (7). Mosquitoes were kept cold on a chilled table during morphologic identification and stored at −80°C. Legs were removed from mosquitoes morphologically identified as Cx. pipiens/Cx. torrentium and used for DNA extraction, enabling identification of the individual specimens to species by using a previously described molecular method (6).
RNA was extracted from 668 mosquito bodies without legs (301 Cx. torrentium, 367 Cx. pipiens) and from 74 pools of mosquitoes pooled by collection trap and week (290 Cs. morsitans); pool sizes ranged from 1 to 19 mosquitoes. The mosquitoes were processed for RNA extraction, real-time reverse transcription PCR (rRT-PCR), and virus isolation on Vero cells as previously described (3).
First, all samples were screened by SINV rRT-PCR by pooling 2–10 RNA extractions by species and collection week. Of 81 total pools, 14 were positive for SINV RNA. Nine positive pools were from Cx. torrentium mosquitoes, and 3 and 2 pools, respectively, were from Cx. pipiens and Cs. morsitans mosquitoes. Second, all individual samples from the SINV-positive pools from the first screening were subjected to another rRT-PCR, so that individual (Culex) or smaller pools of (Culiseta) mosquitoes were ultimately tested for SINV RNA. The second rRT-PCR showed 16 samples positive for SINV RNA. One of the positive Cx. torrentium pools, which contained samples from 10 mosquitoes, included samples from 3 SINV RNA–positive mosquitoes. Thus, 11 of 301 individual Cx. torrentium and 3 of 367 individual Cx. pipiens mosquitoes were positive for SINV RNA. For Cs. morsitans mosquitoes, 2 of 74 pools were positive for SINV RNA (Table 1).
Because individual Cx. torrentium and Cx. pipiens mosquitoes were tested, the IRs were calculated as (no. positive individual mosquitoes/total no. tested) × 1,000; differences between the species were tested for significance by using a χ2 test. The actual species-specific IR differed significantly between species (p = 0.01): 36.5, 8.2, and 21 infections/1,000 mosquitoes for Cx. torrentium, Cx. pipiens, and mixed species (Cx. pipiens and Cx. torrentium), respectively. Cs. morsitans mosquitoes were tested in pools, so we calculated the minimum IR (MIR) as (no. positive pools/total no. mosquitoes tested) × 1,000; the calculated MIR was 6.9 infections/1,000 Cs. morsitans mosquitoes. A comparison of MIR and maximum-likelihood estimates of infection gave similar estimates.
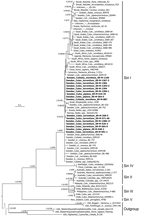
SINV was successfully isolated from all 16 rRT-PCR–positive mosquito samples (Table 2). Part of the SINV E2 envelope glycoprotein gene was sequenced as previously described (8). The phylogenetic analysis included all genomic sequences obtained in this study together with previously published sequences for 63 other virus strains (8). The results showed that all new strains belonged to the SINV-I genotype and have close relationships with strains from Europe, the Middle East, and South Africa (Figure).
We describe information on the actual occurrence and IR of SINV-I in the enzootic mosquito vectors Cx. torrentium and Cx. pipiens, reliably identified to species level. The significantly higher SINV-I IR observed for field-caught Cx. torrentium than Cx. pipiens mosquitoes is a key addition to the previous findings of the extreme susceptibility of Cx. torrentium mosquitoes to SINV-I (4). Experimental transmission studies showed that all infected Cx. torrentium mosquitoes could transmit the virus upon refeeding on a susceptible animal (4). Thus, the observed natural IR of 36.5 infections/1,000 Cx. torrentium mosquitoes translates to 36.0 mosquitoes/1,000 being able to transmit SINV-I. In contrast, because only one third of infected Cx. pipiens mosquitoes can transmit SINV-I upon refeeding, the observed natural IR of 8.2 infections/1,000 Cx. pipiens mosquitoes translates to only 2.0 mosquitoes/1,000 being able to transmit the virus (4).
The observed SINV-I IRs are very high for both species, and the IR for Cx. torrentium is among the highest ever reported for mosquitoes. This could partly be attributed to the fact that single mosquitoes were analyzed, as compared with the more common technique of pooling. This higher IR would have remained undetected if only pooled mosquitoes were analyzed, even though our original pools consisted of only 10 individual mosquitoes. Cx. pipiens mosquitoes are generally considered a secondary enzootic vector of SINV because, as in Sweden, they are less frequently found infected in nature in South Africa, Israel, and Saudi Arabia, where Cx. univittatus mosquitoes are the main SINV vector (9–11).
Because of the exceptionally high SINV IR for field-caught Cx. torrentium mosquitoes in Sweden, the outstanding vector competence results for the species in the laboratory (4), and its status as the dominating Culex species in SINV-endemic areas of Europe (12), Cx. torrentium can now be identified as the main enzootic vector of SINV-I in Scandinavia. In areas of Sweden and Finland where clinical SINV-I infections are most prevalent, Cx. torrentium mosquitoes account for >90% of the Cx. pipiens/Cx. torrentium population; in central Europe, where the virus is more uncommon in mosquitoes and no human cases have been observed, both species are equally common (12–14). Thus, a large population of Cx. torrentium mosquitoes may be a prerequisite for the intense enzootic transmission of SINV-I that is needed to increase the risk for spillover infections in humans. Whether the Cx. torrentium mosquitoes are also to be considered a vector of other mosquitoborne bird viruses remains to be investigated. In continental Europe, West Nile virus and Usutu virus are emerging, and it is unknown if Cx. torrentium has a vector role for these viruses is unknown because of the lack of transmission experiments and isolation attempts from reliably identified female Cx. pipiens/Cx. torrentium mosquitoes. Knowledge from such experiments and isolation attempts would be especially valuable for northern and central Europe where Cx. torrentium is the dominating candidate enzootic vector species for bird-associated viruses (12).
Dr. Hesson is a researcher at Uppsala University in Sweden. Her primary research interests are mosquito-borne human pathogens, especially the effect of mosquito ecology on virus transmission.
Acknowledgments
We are grateful to Pernilla Wahlqvist for collecting and identifying mosquitoes and to the Royal Swedish Academy of Sciences and the Zoological Foundation for financial support.
The phylogenetic computations were performed on resources provided by Swedish National Infrastructure for Computing through Uppsala Multidisciplinary Center for Advanced Computational Science (project p2009050).
References
- Lundström JO. Mosquito-borne viruses in Western Europe: a review. J Vector Ecol. 1999;24:1–39.PubMedGoogle Scholar
- Francy DB, Jaenson TG, Lundström JO, Schildt EB, Espmark Å, Henriksson B, Ecologic studies of mosquitoes and birds as hosts of Ockelbo virus in Sweden and isolation of Inkoo and Batai viruses from mosquitoes. Am J Trop Med Hyg. 1989;41:355–63 .PubMedGoogle Scholar
- Jöst H, Bialonski A, Storch V, Gunther S, Becker N, Schmidt-Chanasit J. Isolation and phylogenetic analysis of Sindbis viruses from mosquitoes in Germany. J Clin Microbiol. 2010;48:1900–3. DOIPubMedGoogle Scholar
- Lundström JO, Niklasson B, Francy DB. Swedish Culex torrentium and Cx. pipiens (Diptera: Culicidae) as experimental vectors of Ockelbo virus. J Med Entomol. 1990;27:561–3. DOIPubMedGoogle Scholar
- Lundström JO, Turell MJ, Niklasson B. Effect of environmental temperature on the vector competence of Culex pipiens and Cx. torrentium for Ockelbo virus. Am J Trop Med Hyg. 1990;43:534–42 .PubMedGoogle Scholar
- Hesson JC, Lundström JO, Halvarsson P, Erixon P, Collado A. A sensitive and reliable restriction enzyme method to distinguish between the mosquitoes Culex torrentium and Culex pipiens. Med Vet Entomol. 2010;24:142–9. DOIPubMedGoogle Scholar
- Lundström JO, Schäfer ML, Hesson JC, Wahlqvist P, Halling A, Hagelin A, The geographic distribution of mosquito species in Sweden. Journal of the European Mosquito Control Association. 2013;31:21–35.
- Lundström JO, Pfeffer M. Phylogeographic structure and evolutionary history of Sindbis virus. Vector Borne Zoonotic Dis. 2010;10:889–907. DOIPubMedGoogle Scholar
- McIntosh BM, Jupp PG, Dos Santos I. Infection by Sindbis and West Nile viruses in wild populations of Culex (Culex) univittatus Theobold (Diptera: Culicidae) in South Africa. Journal of the Entomological Society of South Africa. 1978;41:57–61.
- Samina I, Margalit J, Peleg J. Isolation of viruses from mosquitoes of the Negev, Israel. Trans R Soc Trop Med Hyg. 1986;80:471–2. DOIPubMedGoogle Scholar
- Wills WM, Jacob WL, Francy DB, Oertley RE, Anani E, Calisher CH, Sindbis virus isolations from Saudi Arabian mosquitoes. Trans R Soc Trop Med Hyg. 1985;79:63–6. DOIPubMedGoogle Scholar
- Hesson JC, Rettich F, Merdic E, Vignjević G, Östman Ö, Schäfer M, The arbovirus vector Culex torrentium is more prevalent than the sibling species Culex pipiens in north and central Europe. Med Vet Entomol. 2014;28:179–86. DOIPubMedGoogle Scholar
- Hesson JC, Östman Ö, Schäfer M, Lundström JO. Geographic distribution and relative abundance of the sibling vector species Culex torrentium and Culex pipiens in Sweden. Vector Borne Zoonotic Dis. 2011;11:1383–9. DOIPubMedGoogle Scholar
- Jöst H, Bürck-Kammerer S, Hütter G, Lattwein E, Litzba N, Bock-Hensley O, Medical importance of Sindbis virus in south-west Germany. J Clin Virol. 2011;52:278–9. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 21, Number 5—May 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jenny C. Hesson, Uppsala University, Department of Medical Biochemistry and Microbiology (IMBIM), Zoonosis Science Center, Husargatan 3, Box 582, SE-751 23 Uppsala, Sweden
Top