Volume 21, Number 5—May 2015
CME ACTIVITY - Perspective
Recent US Case of Variant Creutzfeldt-Jakob Disease—Global Implications
Figure 4
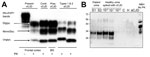
Figure 4. Results of biochemical testing of a US patient with variant Creutzfeldt-Jakob disease (vCJD). A) Immunoblot of vCJD patient and controls. All the PK-treated preparations show similar electrophoretic profiles characterized by 3 bands displaying different mobilities according to number of the linked sugar moieties. The samples from this case and another vCJD case used as positive control (third lane) show the overrepresentation of the diglycosylated band, whereas in sCJD cases, the monoglycosylated band is the most prominent. In both vCJD cases, the unglycosylated band co-migrates with type 2 (sixth lane) as indicated by the type 1 and 2 controls. The PK-untreated preparation (first lane) is used as control of PK digestion. Total brain homogenate, antibody 3F4. B) PrPSc detection in urine by protein misfolding cyclic amplification (PMCA). A urine sample from the patient was processed as previously described (6), and the supernatant fraction was run in duplicate (S1, S2). Samples were subjected to 96 PMCA cycles in the presence of 10% TgHuM brain homogenate, used as the substrate for PMCA. PrPSc signal was assessed by Western blot after PK digestion. As a positive control, vCJD brain homogenate was spiked at 10−5, 10−7, and 10−9 dilutions into urine from a healthy person and processed at the same time and in the same manner for PMCA. Lane C, PMCA-negative control (no sample); lane H, urine from a healthy person; sCJD, urine from an sCJD patient. CJD, Creutzfeldt-Jakob disease; Contr, control; MonoGly, monoglycosylated; NBH, normal brain homogenate without PK treatment used as an electrophoretic migration marker; Pres, present; PK, proteinase K; PrPSc, scrapie prion protein; sCJD, sporadic CJD; Unglyc, unglycosylated.
References
- Prusiner SB. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–3. DOIPubMedGoogle Scholar
- Barria MA, Ironside JW, Head MW. Exploring the zoonotic potential of animal prion diseases: in vivo and in vitro approaches. Prion. 2014;8:85–91. DOIPubMedGoogle Scholar
- Diack AB, Head MW, McCutcheon S, Boyle A, Knight R, Ironside JW, Variant CJD: 18 years of research and surveillance. Prion. 2014;8:286–95. DOIPubMedGoogle Scholar
- Sanchez-Juan P, Cousens SN, Will RG, van Duijn CM. Source of variant Creutzfeldt-Jakob disease outside United Kingdom. Emerg Infect Dis. 2007;13:1166–9. DOIPubMedGoogle Scholar
- Food and Drug Administration. 2011 Meeting materials, Transmissible Spongiform Encephalopthies Advisory Committee [cited 2014 Oct 1]. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/TransmissibleSpongiformEncephalopathiesAdvisoryCommittee/ucm261283.htm
- Moda F, Gambetti P, Notari S, Concha-Marambio L, Catania M, Park K-W, Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N Engl J Med. 2014;371:530–9. DOIPubMedGoogle Scholar
- Heath CA, Cooper SA, Murray K, Lowman A, Henry C, MacLeod MA, Diagnosing variant Creutzfeldt-Jakob disease: a retrospective analysis of the first 150 cases in the UK. J Neurol Neurosurg Psychiatry. 2011;82:646–51. DOIPubMedGoogle Scholar
- Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–5. DOIPubMedGoogle Scholar
- Barash J. Identification of Creutzfeldt-Jakob disease variants. [author reply 1045–6]. Arch Neurol. 2009;66:1045. DOIPubMedGoogle Scholar
- Appleby BS, Appleby KK, Crain BJ, Onyike CU, Wallin MT, Rabins PV. Characteristics of established and proposed sporadic Creutzfeldt-Jakob disease variants. Arch Neurol. 2009;66:208–15 .PubMedGoogle Scholar
- Heath CA, Cooper SA, Murray K, Lowman A, Henry C, MacLeod MA, Validation of diagnostic criteria for variant Creutzfeldt-Jakob disease. Ann Neurol. 2010;67:761–70 .PubMedGoogle Scholar
- World Health Organization Department of Communicable Disease Surveillance and Response. WHO recommended surveillance standards, 1999 [cited 2015 Mar 8]. http://www.who.int/csr/resources/publications/surveillance/whocdscsrisr992.pdf
- Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–68 . DOIPubMedGoogle Scholar
- Collie DA, Summers DM, Sellar RJ, Ironside JW, Cooper S, Zeidler M, Diagnosing variant Creutzfeldt-Jakob disease with the pulvinar sign: MR imaging findings in 86 neuropathologically confirmed cases. AJNR Am J Neuroradiol. 2003;24:1560–9 .PubMedGoogle Scholar
- Ugnon-Café S, Dorey A, Bilheude JM, Streichenberger N, Viennet G, Meyronet D, Rapid screening and confirmatory methods for biochemical diagnosis of human prion disease. J Virol Methods. 2011;175:216–23. DOIPubMedGoogle Scholar
- Hill AF, Butterworth RJ, Joiner S, Jackson G, Rossor MN, Thomas DJ, Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet. 1999;353:183–9. DOIPubMedGoogle Scholar
- Lukic A, Mead S, Rudge P, Collinge J. Comment on validation of diagnostic criteria for variant Creutzfeldt-Jakob disease. [author reply 212–3]. Ann Neurol. 2011;69:212. DOIPubMedGoogle Scholar
- Green AJ, Thompson EJ, Stewart GE, Zeidler M, McKenzie JM, MacLeod MA, Use of 14-3-3 and other brain-specific proteins in CSF in the diagnosis of variant Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2001;70:744–8. DOIPubMedGoogle Scholar
- Zeidler M, Stewart GE, Barraclough CR, Bateman DE, Bates D, Burn DJ, New variant Creutzfeldt-Jakob disease: neurological features and diagnostic tests. Lancet. 1997;350:903–7. DOIPubMedGoogle Scholar
- Beck JA, Poulter M, Campbell TA, Adamson G, Uphill JB, Guerreiro R, PRNP allelic series from 19 years of prion protein gene sequencing at the MRC Prion Unit. Hum Mutat. 2010;31:E1551–63. DOIPubMedGoogle Scholar
- Jackson GS, Burk-Rafel J, Edgeworth JA, Sicilia A, Abdilahi S, Korteweg J, Population screening for variant Creutzfeldt-Jakob disease using a novel blood test: diagnostic accuracy and feasibility study. JAMA Neurol. 2014;71:421–8. DOIPubMedGoogle Scholar
- Edgeworth JA, Farmer M, Sicilia A, Tavares P, Beck J, Campbell T, Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet. 2011;377:487–93. DOIPubMedGoogle Scholar
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46:224–33. DOIPubMedGoogle Scholar
- Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–39. DOIPubMedGoogle Scholar
- Gambetti P, Cali I, Notari S, Kong Q, Zou W-Q, Surewicz WK. Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol. 2011;121:79–90. DOIPubMedGoogle Scholar
- Sikorska B, Knight R, Ironside JW, Liberski PP. Creutzfeldt-Jakob disease. Adv Exp Med Biol. 2012;724:76–90. DOIPubMedGoogle Scholar
- Mihara M, Sugase S, Konaka K, Sugai F, Sato T, Yamamoto Y, The “pulvinar sign” in a case of paraneoplastic limbic encephalitis associated with non-Hodgkin’s lymphoma. J Neurol Neurosurg Psychiatry. 2005;76:882–4. DOIPubMedGoogle Scholar
- Ryan AM, Ryan J, Wan-Ahmed M, Hardiman O, Farrell MA, McNamara B, Vacuolar leucoencephalopathy and pulvinar sign in association with coeliac disease. BMJ Case Rep. 2009;2009:pii: bcr08.2008.0650. PMID: 20414170
- Schmidt C, Plickert S, Summers D, Zerr I. Pulvinar sign in Wernicke’s encephalopathy. CNS Spectr. 2010;15:215–8 .PubMedGoogle Scholar
- US Census Bureau. The foreign-born population in the United States [cited 2014 Aug 27]. https://www.census.gov/newsroom/pdf/cspan_fb_slides.pdf
- Garske T, Ghani AC. Uncertainty in the tail of the variant Creutzfeldt-Jakob disease epidemic in the UK. PLoS ONE. 2010;5:e15626. DOIPubMedGoogle Scholar
- Ghani AC, Donnelly CA, Ferguson NM, Anderson RM. Updated projections of future vCJD deaths in the UK. BMC Infect Dis. 2003;3:4. DOIPubMedGoogle Scholar
- Valleron AJ, Boelle PY, Will R, Cesbron JY. Estimation of epidemic size and incubation time based on age characteristics of vCJD in the United Kingdom. Science. 2001;294:1726–8. DOIPubMedGoogle Scholar
- Brown P, Brandel J-P, Sato T, Nakamura Y, MacKenzie J, Will RG, Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901–7. DOIPubMedGoogle Scholar
- Collinge J, Whitfield J, McKintosh E, Beck J, Mead S, Thomas DJ, Kuru in the 21st century—an acquired human prion disease with very long incubation periods. Lancet. 2006;367:2068–74. DOIPubMedGoogle Scholar
- Boëlle P-Y, Cesbron J-Y, Valleron A-J. Epidemiological evidence of higher susceptibility to vCJD in the young. BMC Infect Dis. 2004;4:26 . DOIPubMedGoogle Scholar
- Gill ON, Spencer Y, Richard-Loendt A, Kelly C, Dabaghian R, Boyes L, Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ. 2013;347:f5675. DOIPubMedGoogle Scholar