Volume 21, Number 6—June 2015
Research
Cost-effectiveness of Chlamydia Vaccination Programs for Young Women
Abstract
We explored potential cost-effectiveness of a chlamydia vaccine for young women in the United States by using a compartmental heterosexual transmission model. We tracked health outcomes (acute infections and sequelae measured in quality-adjusted life-years [QALYs]) and determined incremental cost-effectiveness ratios (ICERs) over a 50-year analytic horizon. We assessed vaccination of 14-year-old girls and catch-up vaccination for 15–24-year-old women in the context of an existing chlamydia screening program and assumed 2 prevaccination prevalences of 3.2% by main analysis and 3.7% by additional analysis. Estimated ICERs of vaccinating 14-year-old girls were $35,300/QALY by main analysis and $16,200/QALY by additional analysis compared with only screening. Catch-up vaccination for 15–24-year-old women resulted in estimated ICERs of $53,200/QALY by main analysis and $26,300/QALY by additional analysis. The ICER was most sensitive to prevaccination prevalence for women, followed by cost of vaccination, duration of vaccine-conferred immunity, and vaccine efficacy. Our results suggest that a successful chlamydia vaccine could be cost-effective.
Chlamydia remains a major public health problem; there were ≈105.7 million new cases of this disease among adults 15–49 years of age worldwide in 2008 (1). In the United States, >1.4 million cases of chlamydial infections were reported to the Centers for Disease Control and Prevention in 2012 (2). A recent study estimated that there were ≈2.8 million cases of chlamydia among all persons of all ages in 2008 (3) and that the estimated direct lifetime cost was >$500 million 2013 US dollars (4). Most infections in women are asymptomatic, and untreated infections can progress to serious sequelae, such as pelvic inflammatory disease (PID), ectopic pregnancy, tubal infertility, and chronic pelvic pain (5,6). In addition, untreated chlamydia may cause serious and costly sequelae, such as urethritis, epididymitis, proctitis, and Reiter syndrome in men (5).
In this study, we explored the health and economic outcomes of a hypothetical chlamydia vaccine in the United States from a societal perspective. Although there currently is no chlamydia vaccine, the future development of an effective chlamydia vaccine is possible, and support for use of a vaccine for future chlamydia prevention efforts continues to increase (7–10). Models of the effect and cost-effectiveness of human papillomavirus (HPV) vaccine were developed before HPV vaccines were approved for use in the United States. These models, as well as subsequent models they helped to inform, proved valuable to public health officials and policy makers (11–14). Our exploratory model is intended to help advance the discussion surrounding development of a successful chlamydia vaccine, to inform the business case for investing in research and development of chlamydia vaccines, and to promote development of more detailed models so that the necessary tools are in place for chlamydia vaccine recommendations.
Model Summary
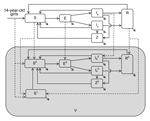
Institutional review board approval was not required for this study because we used only secondary data. To assess the health and economic outcomes of a hypothetical chlamydia vaccine for young persons (15–24 years of age), we accounted for herd effects by using a heterosexual transmission model. We constructed a relatively simple deterministic population-based compartmental model of chlamydia transmission (Figure 1) on the basis of previously published models (15–17). We assumed a population of 100,000 (50% men and 50% women) (13,16). To simplify our model, our population was made up of 1 age group (men and women 15–24 years of age) that has the highest risk for chlamydia infection in the United States (3). Thus, our model was not age-structured.
Given that our model population consisted of 10 birth cohorts (ages 15–24 years), we assumed that annual entry and exit into the population of 15–24-year-old persons was ≈10% of the population. In addition, we assumed that the age at sexual debut (first sexual intercourse) for girls and boys was 15 years. Thus, 14-year-old persons who turned 15 entered the model in susceptible compartments, and 24-year-old persons who turned 25 exited the model at the end of each year, such that the total population was constant at any given time over the analytic horizon (Figure 1). We accounted for heterogeneity in sexual behavior by assuming 2 classes of sexual activity (high and low) on the basis of the annual number of new sex partners. Other details of the model and associated equations are provided in the online Technical Appendix (http://wwwnc.cdc.gov/EID/article/21/6/14-1270-Techapp1.pdf). We assembled data for the model from published reports (Table 1).
Preliminary analyses, as well as results from other cost-effectiveness studies, indicated that the burden of chlamydia was an influential variable. Thus, we conducted 2 analyses: main analysis and additional analysis. In the main analysis, parameter values were selected from within published ranges such that the resulting chlamydia prevalence for women in the model was near the US national average for women 15–24 years of age (i.e., 3.2%) (3) after accounting for the current screening rate of 30%. In the additional analysis, we modified the model by using parameter values from within published ranges of key parameters such that the resulting chlamydia prevalence for women was 0.5% higher than was used in main analysis (i.e., 3.7% and a screening rate of 30%). Specifically, this was achieved by changing the proportion of women and men in the low sexual activity group from 97.9% to 97.6% and from 95.0% to 95.5%, respectively. Essentially, we increased the proportion of women in the high sexual activity group by 0.3% and decreased the proportion of men in the high sexual activity group by 0.5%. These changes were made to provide more information on the resulting health and economic outcomes in a population with a higher chlamydia prevalence.
Vaccine Characteristics
We assumed that vaccine efficacy was 75% at a cost of $547 (2013 US dollars, cost of complete vaccine series per person) and provided immunity for an average of 10 years. As has been performed in most published studies on vaccine cost-effectiveness (8,13,14,28), we repeated the analysis using 100% efficacy and lifelong duration of vaccine immunity. We assumed that the chlamydia vaccine was prophylactic; thus, there were no therapeutic benefits to recipients who were already exposed/infected. We also assumed that persons with symptomatic infections or sequelae were not vaccinated. On the basis of current coverage of HPV vaccine (27), we assumed that chlamydia vaccine coverage for girls 14 years of age and women 15–24 years of age would be 30% achieved by a linear increment during the first 5 years of the onset of the vaccination program and would remain at that rate over the analytic horizon.
Evaluation of Strategies and Health Outcomes
The 4 strategies assessed were A) no screening, no vaccination; B) screening women 15–24 years of age; C) screening women 15–24 years of age and vaccinating girls 14 years of age; and D) screening women 15–24 years of age, vaccinating girls (14-year-old), and catch-up vaccination for women 15–24 years of age. Thus, all persons vaccinated were also subject to annual screening at the same rate as persons who were not vaccinated. For cost purposes, it was assumed that screening would be conducted opportunistically when patients sought other care. Therefore, no productivity costs were assessed for screening.
Health outcomes were measured in quality-adjusted life-years (QALYs) estimated by using health state utility weights for acute infections and sequelae for men (epididymitis) and women (PID), including chronic pelvic pain, ectopic pregnancy, and infertility (16). Cumulative cost and effects (QALYs) were estimated over a 50-year time frame and analytic horizon for all strategies. All outcomes (cost and effects) were discounted at an annual rate of 3%. All costs were adjusted to 2013 US dollars by using the Medical Care component of the Consumer Price Index (29). To provide summaries of cost-effectiveness results from a societal perspective, we included productivity costs in the cost of diseases.
Sensitivity Analyses
We assessed the sensitivity of our results to numerous parameter values (n = 44) that we used in our model. Specifically, we first used the Latin hypercube sampling (4,30) method to create 120 random combinations of parameter values by randomly choosing (without replacement) from 120 equiprobable parameter value intervals from ranges provided in Table 1. To explore all values in specified ranges equally, we assumed uniform distribution for all variables. Next, we ran each simulation and checked to ensure that a steady-state was reached before and after introducing the strategy. We recorded the resulting prevalence (for men and women), costs, and QALYs before and after the vaccination program. We then ranked all values (i.e., parameter values, prevalence and incremental cost-effectiveness ratios [ICERs]) and determined the partial rank correlation coefficients (PRCCs). The PRCCs provided the magnitude of the effect of the referent parameter on the ICER after partially eliminating effects of the other parameters.
In preliminary analyses, we found that prevaccination steady-state prevalence could vary substantially in the sensitivity analyses and that prevaccination prevalence for women was an influential determinant of the effect and cost-effectiveness of the vaccine program. Thus, we divided the PRCC analyses into 2 parts. In the first part, we determined the causal parameters for the prevaccination prevalence and then excluded these parameters from the second and final PRCC analysis, in which we determined the influential variables/parameters of the ICER. Thus, we determined the influential parameters of the prevaccination prevalence for women and included the prevaccination prevalence for women in the second and final PRCC analysis to determine the influential variables/parameters of the ICER. For the sensitivity analyses, we focused on the ICER for strategy C (screen women 15–24 years of age and vaccinate girls 14 years of age) when compared with strategy B (screen women 15–24 years of age).
Main Analysis
In the base-case scenario, chlamydia prevalence in the strategy A scenario (no screening, no vaccination) was 3.73% in women and 2.90% in men. With annual chlamydia screening coverage of 30% (the approximate status quo in the United States), chlamydia prevalence decreased from 3.73% to 3.24% for women and from 2.90% to 2.79% for men (Figure 2). The estimated ICER of strategy B (screen women 15–24 years of age) when compared with strategy A (no screening, no vaccination) was $38,700/QALY gained (Table 2). When vaccinating 14-year-old girls only in addition to screening (i.e., strategy C: screen women 15–24 years of age and vaccinate girls 14 years of age), the chlamydia prevalence was reduced to 2.76% for women and to 2.55% for men, and the estimated ICER of vaccination when compared with the status quo strategy B (i.e., screening 15–24-year-old women) was $35,300/QALY gained (Table 2).
Including catch-up vaccination for 15–24-year-old women (i.e., strategy D, screen women 15–24 years of age, vaccinate girls 14 years of age, and catch-up vaccination for women 15–24 years of age) did not change the long-term reduction in chlamydia prevalence relative to strategy C (Figure 2). However, reductions in chlamydia prevalence were achieved more rapidly than without catch-up vaccination (Figure 2). The estimated ICER of adding catch-up vaccination when compared with strategy C (screen women 15–24 years of age and vaccinate girls 14 years of age) was $53,200/QALY gained. Throughout the analyses, although strategy B was weakly dominated, we did not eliminate it because we wanted to show how vaccine strategies compared with the status quo or existing strategy B (screen females 15–24 years of age).
When we applied values for perfect vaccine performance (i.e., 100% efficacy and lifelong duration of immunity), the chlamydia prevalence in strategy C (screen women 15–24 years of age and vaccinate girls 14 years of age) was reduced further, to 2.01% for women and to 2.14% for men (Figure 2), and the ICER when compared with strategy B (screen women 15–24 years of age) was reduced to $9,700/QALY gained. Adding catch-up vaccination for 15–24 year-old women (i.e., strategy D: screen women 15–24 years of age, vaccinate girls 14 years of age, and catch-up vaccination for women 15–24 years of age) compared with strategy C (screen women 15–24 years of age and vaccinate girls 14 years of age) had an ICER of $16,100/QALY gained (Table 2).
When we assumed perfect chlamydia vaccine performance (i.e., 100% efficacy and lifelong duration of immunity) and increased coverage for 14-year-old persons to >75%, our results indicated that overall illness from chlamydia decreased by ≈ 90% in 20 years. In addition, illness from chlamydia was eliminated in ≈30 years after onset of the vaccination program.
Additional Analysis
Results for additional analysis were similar in relative terms to what we found for main analysis. However, because of higher chlamydia prevalence in additional analysis, the estimated ICERs were substantially lower (<50%) than we found for main analysis (Table 3). When we applied values for perfect vaccine performance (i.e., 100% efficacy and lifelong duration of immunity), the estimated ICER for strategy C (screen women 15–24 years of age and vaccinate girls 14 years of age) was cost-saving (Table 3). Adding a catch-up vaccination program for 15–24-year-old women (i.e., strategy D: screen women 15–24 years of age, vaccinate girls 14 years of age, and catch-up vaccination for women 15–24 years of age) was also highly cost-effective ($1,500/QALY gained over strategy C [screen women 15–24 years of age and vaccinate girls 14-years of age]).
Sensitivity Analyses
A summary of results from the first part of the PRCC analyses used to determine the hierarchy of influential parameters for preintervention prevalence in women is shown in Table 4. Our results indicated that the preintervention prevalence for women was highly sensitive to the proportion of women in the low (or high) sexual activity category, followed by the duration of infection-conferred immunity, per-partner transmission probability (man to woman), duration of asymptomatic infections (woman followed by man), mixing parameter, probability of symptomatic infection (woman followed by man), annual screening coverage (women), number of partners in the past year for women with low sexual activity, number of partners in the past year for women with high sexual activity, number of partners in the past year for men with low sexual activity, duration of symptomatic infections in women, probability of postscreening treatment, and relative size of the population of persons 14 years of age entering the model each year.
The second and final part of the PRCC analyses used to determine the hierarchy of influential parameters/variables of the ICER is shown in Table 4. Our results showed that the most influential variable on the estimated ICER was the prevaccination prevalence in women, followed by 3 vaccine-related variables (vaccine cost, duration of vaccine-conferred immunity, and vaccine efficacy), probability of sequelae in women, and the discount rate.
The estimated prevaccination prevalence for women ranged from 0.06% to 8.51% (mean 2.06%, 95% CI 1.81%–2.31%). The overall average ICER was $86,349/QALY gained (95% CI $66,910–$105,789), but this value was largely attributable to scenarios with low prevalence of chlamydia. When looking at the ICERs for female prevaccination prevalence cutoffs (0.00–1.99, 2.00–3.99, and >4.00), the average ICERs were $125,087/QALY gained (95% CI $94,422–$155,752), $43,037/QALY gained (95% CI $32,824–$53,248), and $4,849/QALY gained (95% CI cost-saving–$28,344], respectively (Figure 3). When prevaccination prevalence for women was 2%–3%, the estimated average ICER was $44,486/QALY gained (95% CI $31,772–$57,202). Finally, when we limited the analysis to include only parameter sets that resulted in chlamydia prevalence within the CIs reported for chlamydia prevalence in the United States (i.e., 2.26%–4.52%) (3), the estimated average ICER was $42,378/QALY gained (95% CI $29,619–$55,136).
We used a deterministic heterosexual transmission model that was relatively simple compared with previously published models (11–14,20,31) to explore the potential health and economic outcomes of a hypothetical chlamydia vaccine focusing on vaccination programs for 14-year-old girls and 15–24-year-old women in the United States. We repeated our analyses by using a higher disease burden. Overall, results from our exploratory analyses showed that a chlamydia vaccine could be cost-effective under many plausible scenarios. Interventions that reduce QALYs lost for <1–3 times per capita gross domestic product (≈$50,000 in the United States) are typically considered to be cost-effective (32). Our sensitivity analyses suggest that a highly efficacious chlamydia vaccine with long duration of immunity might be cost-saving in countries with high prevalence of chlamydia, as demonstrated by results of our additional analysis. Our results are consistent with preliminary, spreadsheet-based calculations, which suggested that a chlamydia vaccine would cost <$10,000/QALY saved (28).
Our analyses showed that a high-performance vaccine could potentially eliminate chlamydia infection when coverage was high (>75%) among susceptible persons before their sexual debut. These results were consistent with findings from previous studies (17,33), and our estimates of cost-effectiveness of chlamydia screening (versus no screening) were consistent with those of previous studies (16,34). In addition, the relative cost-effectiveness of targeting different age groups was consistent with results of previous studies on HPV vaccine (11–14). In particular, our study showed that catch-up vaccination of 15–24-year-old women, in addition to 14-year-old girls, resulted in an increase in the ICER, implying that additional QALYs are gained at higher costs. Consistent with results of Elbasha et al. (13) the addition of catch-up vaccination of 15–24-year-old women did not change the long-term prevalence of infection, but did shorten the time needed to realize the effects of vaccination.
An additional aspect of vaccination is that it is easier to implement than an intervention of routine screening because it does not need to be repeated annually. Although health services data have shown chlamydia screening rates >30% in young women (35), time-series insurance data have shown that <1% of women <25 years of age are consistently screened at least once per year (36).
Our exploratory study has several limitations. Notable among them is the inherent limitations associated with models in general because models are simplifications of real-world events. Thus, all limitations associated with models are applicable. Another major limitation is the high uncertainty surrounding the parameter values we used (including illness estimates). Because we focused on heterosexual transmission, our model was largely driven by parameters associated with women; prevaccination prevalence was calibrated to approximate reported illnesses for women, and prevaccination prevalence for men was determined by the model. Because a substantially high proportion of high-impact health outcomes of chlamydia infection are in women (i.e., PID and associated complications), it is reasonable to focus on illness in women in such analyses. Nonetheless, as was conducted for HPV (11,13), future studies should also assess cost-effectiveness of chlamydia vaccination for men.
The prevaccination prevalence rates for men determined by our model were substantially different from reported prevalence rates for men in the United States. For instance, the reported prevalence for men of a similar age group (15–24 years) in the United States was approximately half that of women (men 1.66%; women 3.21%) (3), and prevalence in men from our main analysis was substantially higher (men 2.79%; women 3.24%).
We excluded numerous possible outcomes of chlamydia vaccination, such as changes in the number of partners or screening practices, which might arise as a result of vaccination, health benefits for persons vaccinated while infected, and costs and loss in quality of life to persons who experience potential adverse vaccination outcomes, such as side effects (e.g., temporary pain at injection site) (11).
We did not explore potential broader properties of an effective chlamydia vaccine, such as degree (i.e., reducing susceptibility but not completely eliminating it) or infectiousness (i.e., breakthrough infections being less infectious than primary infections and shorter in duration). Future studies should consider assessing these 2 characteristics (degree and infectiousness). We assumed that all members of the hypothetical population (with substantially different sexual activity levels) have equal access to screening, treatment, and vaccination. Thus, treatment rate, screening rate, and vaccination coverage were applied equally across all eligible model compartments (subpopulations). However, this simplifying assumption is probably not realistic. Consequently, benefits of screening and vaccination might have been overestimated if women who are highly sexually active were less likely to be screened, treated, or vaccinated. In addition, it is also conceivable that persons vaccinated might be less likely to be screened for chlamydia annually. Further studies are needed to explore the potential health and economic benefits of a chlamydia vaccine that targets specific subpopulations, such as persons infected, those with limited access to health care, and those who have multiple sexual partners.
Because our model does not account for major factors, such as age-based mixing of sexual partners and ongoing sexual partnerships, our model is not of sufficient complexity to inform chlamydia vaccine recommendations. For example, our model assumed sexual debut at 15 years of age and that sex partners were chosen from a pool of 15–24 year-old persons, thereby ignoring heterogeneity in age at sexual debut, which is a simplification (37). Similarly, models such as ours that do not specifically keep track of ongoing sexual partnerships can overestimate the effect of chlamydia screening because reinfection of treated women by their untreated sex partner is not specifically taken into account (38,39). If the effect of chlamydia screening is overestimated, then the marginal effect of adding chlamydia vaccination to an existing chlamydia screening program might be underestimated. Development of more complex models will be needed over time, and these models would be better suited to examine the effect of vaccination over a wide range of assumptions regarding vaccine coverage, efficacy, and duration of protection.
Notwithstanding these limitations, our model provides useful information on the potential cost-effectiveness of a chlamydia vaccine, as well as a useful basis for future chlamydia vaccine cost-effectiveness analyses and other modeling studies. In particular, determination of the hierarchy of influential parameters in our model would be useful for future analyses, and assist in understanding the relative roles played by numerous variables that are used in models to facilitate discussions around simple and complex model inputs. Finally, our study suggests that a successful chlamydia vaccine could have a substantial effect on chlamydia prevalence, thereby reducing the health and economic burden associated with chlamydia.
Dr. Owusu-Edusei is an economist at the Centers for Disease Control and Prevention, Atlanta, GA. His primary interests include cost and cost-effectiveness analysis of sexually transmitted infection interventions and policies.
References
- World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections, 2008. Geneva: The Organization; 2012.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2012. Atlanta: The Centers; 2013.
- Satterwhite CL, Torrone E, Meitis E, Dunne EF, Mahajan R, Ocfemia MC, Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–93. DOIPubMedGoogle Scholar
- Owusu-Edusei K Jr, Chesson HW, Gift TL, Tao G, Ocfemia MC, Mahajan R, The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40:197–201. DOIPubMedGoogle Scholar
- Stamm WE. Chlamydia trachomatis infections in the adult. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, et al., editors. Sexually transmitted diseases. New York: McGraw Hill; 2008. p. 575–93.
- Bakken IJ, Skjeldestad FE, Nordbo SA. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: A population-based, nested case-control study. Sex Transm Dis. 2007;34:166–9. DOIPubMedGoogle Scholar
- Hafner LM, Wilson DP, Timms P. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine. 2014;32:1563–71. DOIPubMedGoogle Scholar
- Brunham RC, Rappuoli R. Chlamydia trachomatis control requires a vaccine. Vaccine. 2013;31:1892–7. DOIPubMedGoogle Scholar
- Geisler WM, Morrison SG, Doemland ML, Iqbal SM, Su J, Mancevski A, Immunoglobulin-specific responses to Chlamydia elementary bodies in individuals with and at risk for genital chlamydial infection. J Infect Dis. 2012;206:1836–43. DOIPubMedGoogle Scholar
- Gottlieb SL, Low N, Newman LM, Bolan G, Kamb M, Broutet N. Toward global prevention of sexually transmitted infections (STIs): the need for STI vaccines. Vaccine. 2014;32:1527–35. DOIPubMedGoogle Scholar
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29:8443–50. DOIPubMedGoogle Scholar
- Chesson HW, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of human papillomavirus vaccination in the United States. Emerg Infect Dis. 2008;14:244–51. DOIPubMedGoogle Scholar
- Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13:28–41. DOIPubMedGoogle Scholar
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359:821–32. DOIPubMedGoogle Scholar
- Owusu-Edusei K Jr, Gift TL, Chesson HW, Kent CK. Investigating the potential public health benefit of jail-based screening and treatment programs for Chlamydia. Am J Epidemiol. 2013;177:463–73. DOIPubMedGoogle Scholar
- Gift TL, Gaydos CA, Kent CK, Marrazzo JM, Rietmeijer CA, Schillinger JA, The program cost and cost-effectiveness of screening men for Chlamydia to prevent pelvic inflammatory disease in women. Sex Transm Dis. 2008;35(Suppl):S66–75. DOIPubMedGoogle Scholar
- Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–44. DOIPubMedGoogle Scholar
- Price MJ, Ades AE, De Angelis D, Welton NJ, Macleod J, Soldan K, Risk of pelvic inflammatory disease following Chlamydia trachomatis infection: analysis of prospective studies with a multistate model. Am J Epidemiol. 2013;178:484–92. DOIPubMedGoogle Scholar
- Quinn TC, Gaydos C, Shepherd M, Bobo L, Hook EW, Viscidi R, Epidemiologic and microbiologic correlates of Chlamydia trachomatis infection in sexual partnerships. JAMA. 1996;276:1737–42. DOIPubMedGoogle Scholar
- Garnett GP, Mertz KJ, Finelli L, Levine WC, St. Louis ME. The transmission dynamics of gonorrhoea: modelling the reported behaviour of infected patients from Newark, New Jersey. Philos Trans R Soc Lond B Biol Sci. 1999;354:787–97. DOIPubMedGoogle Scholar
- Van Der Pol B, Liesenfeld O, Williams JA, Taylor SN, Lillis RA, Body BA, Performance of the cobas CT/NG test compared to the Aptima AC2 and Viper CTQ/GCQ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2012;50:2244–9. DOIPubMedGoogle Scholar
- Geisler WM. Management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: evidence reviewed for the 2006 Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2007;44(Suppl 3):S77–83. DOIPubMedGoogle Scholar
- Owusu-Edusei K Jr, Nguyen HT, Gift TL. Utilization and cost of diagnostic methods for sexually transmitted infection screening among insured American youth, 2008. Sex Transm Dis. 2013;40:354–61 . DOIPubMedGoogle Scholar
- Owusu-Edusei K, Roby TM, Chesson HW, Gift TL. Productivity costs of nonviral sexually transmissible infections among patients who miss work to seek medical care: evidence from claims data. Sex Health. 2013;10:434–7. DOIPubMedGoogle Scholar
- Bureau of Labor Statistics. The editor’s desk. Youth employment and unemployment in July 2010, 2013 [cited 2013 Sep 15]. http://www.bls.gov/opub/ted/2010/ted_20100903.htm
- Blandford JM, Gift TL. Productivity losses attributable to untreated chlamydial infection and associated pelvic inflammatory disease in reproductive-aged women. Sex Transm Dis. 2006;33(Suppl):S117–21. DOIPubMedGoogle Scholar
- Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–93. DOIPubMedGoogle Scholar
- Institute of Medicine. Vaccines for the 21st century: a tool for decisionmaking. Washington (DC): National Academy of Sciences; 2000.
- United States Department of Labor. Consumer price indexes—all urban consumers. 2011 [cited 2013 Dec 15]. http://www.bls.gov/cpi/home.htm
- Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model as an example. Int Stat Rev. 1994;62:229–43. DOIGoogle Scholar
- Chen MI, Ghani AC, Edmunds WJ. A metapopulation modelling framework for gonorrhoea and other sexually transmitted infections in heterosexual populations. J R Soc Interface. 2009;6:775–91 . DOIPubMedGoogle Scholar
- Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–78.
- Gray RT, Beagley KW, Timms P, Wilson DP. Modeling the impact of potential vaccines on epidemics of sexually transmitted Chlamydia trachomatis infection. J Infect Dis. 2009;199:1680–8. DOIPubMedGoogle Scholar
- Hu D, Hook EW III, Goldie SJ. Screening for Chlamydia trachomatis in women 15 to 29 years of age: a cost-effectiveness analysis. Ann Intern Med. 2004;141:501–13. DOIPubMedGoogle Scholar
- National Commission for Quality Assurance. Improving quality and patient experience: the state of health care quality. Washington (DC): The Commission; 2013.
- Heijne JCM, Tao GY, Kent CK, Low N. Uptake of regular Chlamydia testing by US women: a longitudinal study. Am J Prev Med. 2010;39:243–50. DOIPubMedGoogle Scholar
- Finer LB, Philbin JM. Sexual initiation, contraceptive use, and pregnancy among young adolescents. Pediatrics. 2013;131:886–91. DOIPubMedGoogle Scholar
- Althaus CL, Heijne JC, Roellin A, Low N. Transmission dynamics of Chlamydia trachomatis affect the impact of screening programmes. Epidemics. 2010;2:123–31.
- Low N, Heijne JC, Kretzschmar M. Use of mathematical modeling to inform Chlamydia screening policy decisions. J Infect Dis. 2009;199:767–8. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 21, Number 6—June 2015
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Kwame Owusu-Edusei Jr. Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop E80, Atlanta, GA 30329-4027, USA
Top