Volume 22, Number 10—October 2016
Dispatch
Hypervirulent Clone of Group B Streptococcus Serotype III Sequence Type 283, Hong Kong, 1993–2012
Abstract
We describe a hypervirulent clone of group B Streptococcus serotype III, subtype 4, sequence type 283, that caused invasive disease with a predilection for meningitis in Hong Kong during 1993–2012. The organism is associated with high mortality and increased summer prevalence and is linked to diseased fish from freshwater fish farms.
Group B Streptococcus (GBS) has been implicated in neonatal sepsis and infections in pregnant women. Worldwide, the predominant clone belongs to serotype III, subtype 2 (III-2), sequence type (ST) 17 (1), which is less genetically diverse and heterogeneous than strains from the other serotypes (Ia, Ib, II–IX). In July 2015, the Singapore Ministry of Health reported an outbreak of GBS invasive disease in adults and suspected a link to raw fish consumption (2). The Ministry subsequently reported that this GBS belonged to ST283 (3), and its whole genome has been recently sequenced (4). GBS strains of ST283 were linked to a series of adult meningitis cases in Singapore and Hong Kong dating from 1998 (5). In addition, GBS III-4/ST283 is a hypervirulent lineage that has been associated with invasive GBS disease in nonpregnant adults (6). We investigated the epidemiology of GBS infections caused by GBS III-4/ST283 during 1993–2012 in Hong Kong and examined possible associations with seasons, temperature, and humidity. We also compared the invasive potential of different GBS serotypes among neonates, pregnant women, and nonpregnant adults.
We investigated GBS serotypes found among 1,645 isolates from patients admitted to a university hospital in Hong Kong during 1993–2012. Of the isolates, 437 were invasive GBS (i.e., isolated from sterile blood, cerebrospinal fluid, and body fluid specimens), and 1,208 were noninvasive (Technical Appendix Table). We stratified these patients into 3 groups: 249 neonates, 704 pregnant women, and 692 nonpregnant adults (women and adults were >16 years of age). We used PCR, pulsed-field gel electrophoresis, and multilocus sequence typing for serotyping and subtyping of the isolates, as described (1,6). We reviewed demographic and medical records for the 1,645 patients and performed statistical analysis by using Fisher exact and χ2 tests. SPSS version 19 (IBM, Armonk, New York, USA) was used for Spearman correlation analysis. We obtained ethical approval from the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (CRE-2012.054).
Using an empirical odds ratio (OR) (7), we compared the invasive potential of serotype III-4 isolates with that of all other serotypes (Table 1). Serotypes III-2 (n = 76; OR 1.5, 95% CI 1.1–2.1; p<0.01) and III-4 (n = 50; OR 19.4, 95% CI 9.1–41.2; p<0.001) had significantly higher (OR >1.0) invasive potential than other serotypes. Serotypes III-2 was the predominant cause of invasive disease in neonates (OR 5.3, 95% CI 2.7–10.3; p<0.001). Serotype III-4 was highly invasive in nonpregnant adults (OR 18.4, 95% CI 7.7–43.9; p<0.001) and in neonates (OR 6.3, 95% CI 0.7–54.3; p<0.01).
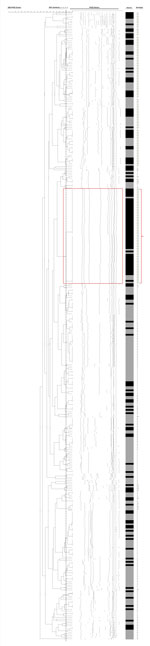
By using pulsed-field gel electrophoresis, all 48 serotype III-4 strains showed indistinguishable patterns and were confirmed as GBS III-4/ST283 (Figure 1). Medical records for these 48 patients (44 nonpregnant adults, 3 neonates, and 1 pregnant woman) revealed that invasive disease has been identified in nonpregnant adults since 1995 and invasive illness in neonates and pregnant women since 2009.
Mean age of the 44 nonpregnant adults with GBS III-4/ST283 infection was 63 (range 23–96) years; half were >65 years of age. Twenty-eight (63.6%) of the 44 patients had underlying diseases (e.g., diabetes, hypertension, malignancy, chronic rheumatic heart, and gout). Serious complications developed in 35 (80%) of the 44 nonpregnant adult patients: 17 (38.6%) had sepsis, 10 (22.7%) had septic arthritis, 7 (15.9%) had meningitis, and 2 (4.5%) had infective endocarditis. Twelve (27.3%) patients died. For patients >65 years of age, presence of underlying diseases or serious complications was not statistically associated with fatal outcome (p>0.05).
Of the 44 nonpregnant adults with GBS III-4/ST283, 41 (93.2%) had isolates from invasive sites. Sex of patients had no effect on whether the GBS disease was invasive: 20 of these patients were women and 21 were men.
Among the total 227 nonpregnant adults with invasive GBS infection, 12 (5.5%) had meningitis (Table 2). Of these patients, 7 (58.3%) were infected with serotype III-4 alone. Compared with other GBS serotypes, GBS III-4 had significantly higher potential (p<0.001 by χ2 test) to cause meningitis in nonpregnant adults.
Among the 3 neonates with invasive disease caused by GBS III-4/ST283, one infant had early-onset sepsis, and 2 had late-onset sepsis (1 with meningitis). Thirty neonates had meningitis, 16 (53.3%) caused by serotype III-2 and 1 (3.3%) caused by serotype III-4.
To assess contributing factors, we examined possible climatic associations with isolation of serotypes causing the 437 cases of invasive GBS disease in Hong Kong during our study period. We reviewed Hong Kong’s average monthly temperature and humidity records for 1997–2012. Serotype III-4 was the only serotype with a prevalence significantly associated with monthly temperature (Spearman correlation r = 0.622; p = 0.031). Isolation of serotype III-4 peaked during the summer months (June–September), when mean temperature was >28°C) (Figure 2, panel A). Humidity was not significantly associated with serotype III-4 prevalence (p>0.05) (Figure 2, panel B).
GBS infects many host species, has become an important pathogen in the aquaculture industry, and has resulted in significant economic loss (8). Serotype Ia/ST7 has been associated with large outbreaks of streptococcosis in cultured tilapia in China (9). The pathogenicity of human ST7 isolates in fish has been well established (10). Although no epidemiologic evidence confirms GBS as a zoonosis in human infections (11), comparative genome analysis revealed that cultured tilapia GBS ST7 was closely related to human ST7 strain A909. These fish and human strains share highly similar clustered regularly interspaced short palindromic repeats, prophages, virulence-associated genes, and extremely short evolutionary relationships in phylogenetic analysis (12).
ST283 and its single-locus variant ST491 have been detected in diseased tilapia with high death rates in Southeast Asia, where fish isolates shared the same mobile genetic elements and surface proteins as the human isolates described in this article (8,13). This similarity provides molecular evidence for the linkage of ST283 strains in humans and fish. The outbreak in Singapore showed a rise in GBS sepsis and septic arthritis, which were linked to a probable source of raw fish consumption; subsequently, health authorities advised discontinuing sales of the raw fish dish as a precaution (2). A previous study showed that fish consumption caused a 7.3-fold increased risk for acquiring GBS serotype Ia and Ib colonization in humans (14). GBS serotypes Ia, III, and V of clonal complexes (CC) 19, CC23, and CC103 from a human and a cow have been shown to infect tilapia experimentally (15); these cross-infections support GBS pathogenicity across different species. Epidemiologic investigations and comparative genomics on a wider scale of animal and human strains may further reveal evolutionary relationships between these GBS lineages.
Our finding of an association of the GBS III-4/ST283 lineage with summer months coincides with the timing of the outbreak in Singapore. Because GBS grows rapidly in warm temperatures, a higher infective dose may occur in warmer months than at other times. Warm temperatures might also cause increased pathogenicity of this organism, a postulation supported by the association of higher temperatures with tilapia deaths caused by GBS III-4 in freshwater fish farms (13). The pathogenicity of this strain in fish has been shown in vivo and further highlights the pathogenic potential of this lineage.
Reports dating from the 1990s indicate that GBS ST283 isolated from humans came exclusively from invasive sites in nonpregnant adults. Our data suggest that these isolates might have undergone adaptive evolution in recent years to colonize and invade neonates with early- and late-onset GBS sepsis. Studies addressing the changing epidemiology of this hypervirulent lineage and its relationship to humans and fish are warranted to reduce potential transmission between the 2 hosts.
Dr. Ip is a clinical microbiologist at the Chinese University of Hong Kong and works in bacteriology in the Department of Microbiology. Her research interests include clinical and molecular epidemiology of gram-positive cocci (e.g., methicillin-resistant Staphylococcus aureus, Group B Streptococcus, and Streptococcus pneumoniae) and antimicrobial resistance determinants of Streptococcus pneumoniae and other pathogenic bacteria.
Acknowledgment
This work was supported by a grant from the Health and Medical Research Fund of the Food and Health Bureau, Hong Kong Special Administrative Region (project no. 12110612).
References
- Zhao Z, Kong F, Zeng X, Gidding HF, Morgan J, Gilbert GL. Distribution of genotypes and antibiotic resistance genes among invasive Streptococcus agalactiae (group B streptococcus) isolates from Australasian patients belonging to different age groups. Clin Microbiol Infect. 2008;14:260–7. DOIPubMedGoogle Scholar
- Government of Singapore. News. Group B Streptococcus investigations update. 2015 July 24 [cited 2015 Aug 26]. http://www.gov.sg/news/content/group-b-streptococcus-investigations-update
- Singapore Ministry of Health. National Environment Agency. Agri-food & Veterinary Authority of Singapore. Advisory issued on consumption of raw fish. 2015 Nov 27 [cited 2016 May 8]. https://www.moh.gov.sg/content/moh_web/home/pressRoom/pressRoomItemRelease/2015/advisory-issued-on-consumption-of-raw-fish.html
- Mehershahi KS, Hsu LY, Koh TH, Chen SL. Complete genome sequence of Streptococcus agalactiae serotype III, multilocus sequence type 283 strain SG-M1. Genome Announc. 2015;3:e01188-–15.http://dx.doi/10.1128/genomeA.01188-15PubMedGoogle Scholar
- Wilder-Smith E, Chow KM, Kay R, Ip M, Tee N. Group B streptococcal meningitis in adults: recent increase in Southeast Asia. Aust N Z J Med. 2000;30:462–5. DOIPubMedGoogle Scholar
- Ip M, Cheuk ES, Tsui MH, Kong F, Leung TN, Gilbert GL. Identification of a Streptococcus agalactiae serotype III subtype 4 clone in association with adult invasive disease in Hong Kong. J Clin Microbiol. 2006;44:4252–4. DOIPubMedGoogle Scholar
- Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. 2003;187:1424–32. DOIPubMedGoogle Scholar
- Delannoy CM, Crumlish M, Fontaine MC, Pollock J, Foster G, Dagleish MP, Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol. 2013;13:41. DOIPubMedGoogle Scholar
- Zhang D, Li A, Guo Y, Zhang Q, Chen X, Gong X. Molecular characterization of Streptococcus agalactiae in diseased farmed tilapia in China. Aquaculture. 2013;412–413:64–9. DOIGoogle Scholar
- Evans JJ, Klesius PH, Pasnik DJ, Bohnsack JF. Human Streptococcus agalactiae isolate in Nile tilapia (Oreochromis niloticus). Emerg Infect Dis. 2009;15:774–6. DOIPubMedGoogle Scholar
- Gauthier DT. Bacterial zoonoses of fishes: a review and appraisal of evidence for linkages between fish and human infections. Vet J. 2015;203:27–35. DOIPubMedGoogle Scholar
- Liu G, Zhang W, Lu C. Comparative genomics analysis of Streptococcus agalactiae reveals that isolates from cultured tilapia in China are closely related to the human strain A909. BMC Genomics. 2013;14:775. DOIPubMedGoogle Scholar
- Suanyuk N, Kong F, Ko D, Gilbert GL, Supamattaya K. Occurrence of rare genotypes of Streptococcus agalactiae in cultured red tilapia Oreochromis sp. and Nile tilapia O. niloticus in Thailand—relationship to human isolates? Aquaculture. 2008;284:35–40. DOIGoogle Scholar
- Foxman B, Gillespie BW, Manning SD, Marrs CF. Risk factors for group B streptococcal colonization: potential for different transmission systems by capsular type. Ann Epidemiol. 2007;17:854–62. DOIPubMedGoogle Scholar
- Chen M, Wang R, Luo F-G, Huang Y, Liang W-W, Huang T, Streptococcus agalactiae isolates of serotypes Ia, III and V from human and cow are able to infect tilapia. Vet Microbiol. 2015;180:129–35. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 22, Number 10—October 2016
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Margaret Ip, Department of Microbiology, Chinese University of Hong Kong, The Prince of Wales Hospital, 30-32 Ngan Shing St, Sha Tin, New Territories, Hong Kong
Top