Volume 22, Number 11—November 2016
Research
Epidemiology of La Crosse Virus Emergence, Appalachia Region, United States
Abstract
La Crosse encephalitis is a viral disease that has emerged in new locations across the Appalachian region of the United States. Conventional wisdom suggests that ongoing emergence of La Crosse virus (LACV) could stem from the invasive Asian tiger (Aedes albopictus) mosquito. Efforts to prove this, however, are complicated by the numerous transmission routes and species interactions involved in LACV dynamics. To analyze LACV transmission by Asian tiger mosquitoes, we constructed epidemiologic models. These models accurately predict empirical infection rates. They do not, however, support the hypothesis that Asian tiger mosquitoes are responsible for the recent emergence of LACV at new foci. Consequently, we conclude that other factors, including different invasive mosquitoes, changes in climate variables, or changes in wildlife densities, should be considered as alternative explanations for recent increases in La Crosse encephalitis.
In recent years, several vectorborne diseases have reemerged either at new locations or to new levels in locations where they have historically ranged. Commonly cited factors for reemergence include evolution of novel vector or pathogen strains (1), increased human mobility or disease spread by infected travelers, decreased herd immunity (2), landscape change (3), climate change (4), and invasion of new regions by competent disease vectors (5). Although disease translocations across continents are almost always a result of human transport, pathogens that exhibit novel regional spread, increased transmission in preexisting locations, or both are more difficult to explain. Such is the case with La Crosse encephalitis, a mosquitoborne viral disease currently emerging in the US Appalachian region (Appalachia, comprising Tennessee, North Carolina, Virginia, and West Virginia). With 30–180 cases of severe LACV disease reported annually (6) and an estimated total disease annual incidence as high as 300,000 cases, LACV is rapidly becoming a leading cause of encephalitis in the United States (7,8). For patients with severe cases, LACV disease has lifelong neurologic consequences (6) and carries an estimated fatality rate of 0.5%–1.9% (6,9).
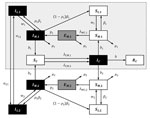
Previously, most LACV disease cases were associated with forested areas in the midwestern United States (10), where LACV was maintained through a cycle involving the eastern tree-hole mosquito (Ochlerotatus triseriatus), hereafter called the tree-hole mosquito, and mammals of 3 species: eastern chipmunks (Tamias striatus), gray squirrels (Sciurus carolinensis), and fox squirrels (Sciurus niger) (10,11). However, since the mid-1990s, Appalachia has emerged as a new focus for the disease (8,12–14). One potential explanation is the introduction of the invasive Asian tiger mosquito (Aedes albopictus), hereafter called the tiger mosquito (15). This suggestion is based on the laboratory-demonstrated competence of the tiger mosquito (16,17), isolation of LACV from field-collected tiger mosquito pools (18), observation of LACV-positive tiger mosquitoes at sites of LACV infections of humans (19), and the coincidental link between tiger mosquito invasion and the emergence of LACV in the Appalachian region (12). Unfortunately, although these observations demonstrate the potential for the tiger mosquito to influence LACV dynamics, the contribution of this mosquito to observed increases in LACV transmission remains unclear. One obstacle to identifying the role of the tiger mosquito in LACV emergence is our limited understanding of the interaction between invasive species and native disease cycles and how this interaction affects disease transmission, both within natural reservoirs and to human hosts. Epidemiologic modeling is a powerful tool, useful for understanding the outcomes of different transmission pathways in other disease systems. To our knowledge, however, no dynamic models for LACV disease have been developed, even for regions where the tree-hole mosquito is the only disease vector. We therefore developed a series of compartmental models (Figure 1) for LACV. Using these models, we then explored LACV dynamics in systems with (native) and without (invaded) tiger mosquitoes to assess the likelihood that the tiger mosquito is responsible for the emergence of LACV in Appalachia.
Model
We built 3 models: 1) the tree-hole model, in which the tree-hole mosquito is the only LACV vector; 2) the tiger model, in which the tiger mosquito is the only LACV vector; and 3) the tree-hole/tiger model, in which mosquitoes of both species simultaneously serve as LACV vectors. In the third model, mosquitoes of either species may be driven extinct through competitive exclusion; thus, although both vectors are potentially present, it is possible that only 1 persists. For all models, we assumed that the vertebrate host was the eastern chipmunk. The basic dynamic system (Figure 1), including all relevant assumptions and system parameterizations, is fully described elsewhere (see Basic Model, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html).
Basic Reproduction Number, R0
To determine whether sustained LACV transmission is predicted, we considered the basic reproduction number, R0, for each of the 3 models (see Basic Reproduction Numbers, R0, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html). For R0>1, LACV transmission can be sustained. For R0<1, LACV will go extinct.
Transmission Pathways
The tree-hole/tiger model, which represents current LACV spread throughout much of Appalachia and the Midwest, accounts for 2 vector species and, thus, 4 transmission pathways: 1) horizontal transmission between tree-hole mosquitoes and chipmunks, 2) vertical (transovarial) transmission by tree-hole mosquitoes, 3) horizontal transmission between tiger mosquitoes and chipmunks, and 4) vertical transmission by tiger mosquitoes. To determine the relative contributions of the different transmission pathways to R0, we used elasticity analyses (20). Large elasticities indicate transmission routes that contribute most to disease maintenance and spread (see Elasticity Analysis of Transmission Pathways, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html).
LAC Dynamics
R0 analyses reflect equilibrium conditions, which are good approximations of full system behavior when seasonality is weak or when the system reaches equilibrium rapidly within a single season. Because the relevance of seasonality for LACV is unknown, we also considered fully dynamic multiseason extensions to each of our models (see LAC Dynamics, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html). Using dynamic simulations, we estimated the fraction of scenarios (i.e., parameter combinations) resulting in sustained LACV transmission above a critical threshold. This is the numerical equivalent of R0 but may differ as a result of seasonality. For dynamic simulations in which LACV persists, we also quantified season-long host seroprevalence rates, peak rates of mosquito infection, and the timing of peak transmission to humans. Last, we considered the potential for the tiger mosquito to act as a bridge vector, linking LACV transmission in wildlife reservoirs to infections in human populations.
Latin Hypercube Sampling
Measurements of system parameters vary, for example, as a result of geographic differences in abiotic variables, differences in local mosquito or chipmunk populations, differences in circulating virus strains, or measurement error. To capture model predictions over empirically determined parameter ranges, we used Latin hypercube sampling, followed by sensitivity analyses with partial rank correlation coefficients (PRCCs) (see Latin Hypercube Sampling and PRCC, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html) (21).
Basic Reproduction Number, R0
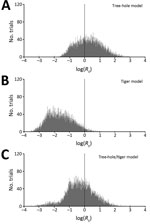
We found that sustained LACV transmission can occur according to most (60%) tree-hole model scenarios but only a small fraction (3%) of tiger model scenarios (Figure 2) (see Latin Hypercube Sampling and PRCC, and Tables A2 A3, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html). This finding is surprising because the average tiger mosquito population has approximately twice as many biting females per hectare as does the average tree-hole mosquito population (see Table A2, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html), which reflects the higher larval carrying capacity and faster larval maturation rate of tiger mosquitoes than those of tree-hole mosquitoes. Clearly, the numerical abundance of tiger mosquitoes does not compensate for the lower rates of horizontal and vertical LACV transmission and the lower rates of their biting on key host species (see Basic Model, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html).
In the 2-vector system, our results for the tree-hole/tiger model indicated a similar outcome—that the invasion of tiger mosquitoes into tree-hole mosquito populations should reduce the fraction of scenarios (from 60% to 37%) in which LACV transmission is viable. Thus, instead of causing the emergence of new LACV foci, tiger mosquitoes should instead drive LACV out of regions where previously it could persist. This result is again a function of the poor intrinsic capability of tiger mosquitoes to serve as LACV vectors. It also depends on asymmetric competition between tiger and tree-hole mosquitoes (see Latin Hypercube Sampling and PRCC and Table A3, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html). For example, whereas 14% of parameter combinations yielded tiger mosquitoes competitively excluding tree-hole mosquitoes, for only 0.03% of parameter combinations was the converse true. Moreover, even when tiger and tree-hole mosquitoes were predicted to coexist, the tree-hole mosquito population declined by an average of 63% through interspecific competition. By contrast, interspecific competition reduced the tiger mosquito population by an average of only 16%. Not surprisingly, then, when both mosquito species were present, most (average 78%) were tiger mosquitoes. Because the tiger mosquito is the less competent of the 2 LACV vectors, its invasion actually reduces the likelihood of LACV transmission.
Transmission Pathways
Elasticity analysis of the 4 virus transmission pathways in the tree-hole/tiger model indicated that in most scenarios the pathway that contributes most to disease spread is horizontal transmission by tree-hole mosquitoes (Figure 3). Vertical transmission by tree-hole mosquitoes can also contribute but usually only when the role of tiger mosquitoes is minimal. For scenarios in which tiger mosquitoes contribute to spread, the main pathway is horizontal transmission either by tiger mosquitoes alone or in combination with horizontal transmission by tree-hole mosquitoes. By contrast, vertical transmission by tiger mosquitoes rarely affects disease dynamics; when it does, it is only in systems in which horizontal transmission by tiger mosquitoes is already the major mode of disease spread.
LACV Dynamics
Predictions from dynamic models were similar to predictions for R0 (LACV transmission in 46% of scenarios) and matched many expectations from LACV systems (Table; see also LAC Dynamics at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html). First, in the native system (the tree-hole model) and the invaded system (the tree-hole/tiger model), predicted host seroprevalence rates were remarkably high, approaching 100% toward the end of the season (mean [median] end-of-season host seroprevalence rates of 89% [99%] in the tree-hole model and 84% [97%] in the tree-hole/tiger model). These rates are consistent with findings from Wisconsin where, at least in high-quality mosquito habitats, multiple surveys have demonstrated that antibody prevalence rates among chipmunks can be well over 50%, often nearing 100% late in the season (15,22). At the same time, our model predicted very low numbers of LACV-positive mosquitoes, even in the native system (mean [median] yearly averages of 2.0% [1.6%] for the tree-hole model). Again, this finding is highly consistent with observed minimum field infection rates that range from 0.26 to 12.5 (14,23–26). Of note, predicted rates of infection among overwintering eggs were even lower than rates of infection among adult populations (mean [median] end-of-season infection rates of 0.63% [0.49%] for the tree-hole model.) This finding reflects the fact that transovarial transmission is <100% and that overwintering eggs are laid later in the season, sometimes after peak LACV transmission has subsided. Again, predicted rates of egg infection strongly agree with field data indicating that 0.29%–0.6% of overwintering eggs from LACV-endemic sites yield LACV-positive larvae (26,27). Last, our predicted timing of peak risk for human disease was broadly consistent with observed cases of LACV disease in humans that tend to occur in late summer and early fall (8, 13).
What our dynamic model did not predict was any increase in LACV prevalence in the invaded system (tree-hole/tiger model) over that in the native system (tree-hole model). Even in systems in which LACV survived introduction of the tiger mosquito, the tiger mosquito tended to decrease LACV transmission. For example, both the absolute number of infected mosquitoes and the rate of mosquito infection were lower in the tree-hole/tiger model than in the tree-hole model (Table). Consistent with rates of mosquito infection, we found that rates of host seroprevalence were also lower when tiger mosquitoes were present.
Although the tiger mosquito is a poor amplifying vector for LACV, it may still increase the number of human LACV infections. Indeed, because this species is an aggressive human biter, it has the potential to intensify the rate of disease transfer to human populations, albeit while simultaneously reducing disease spread in wildlife reservoirs (i.e., it may act as a bridge vector). However, this potential is not realized (Table). Although rates at which tiger mosquitoes bite humans (see Basic Model, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html) partially compensated for lower rates of LACV transmission among wildlife reservoirs, this compensation was not complete. Thus, human infections were still predicted to occur more commonly in the native system (Table; see also Summary Statistics for Alternate Scenarios at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html).
Sensitivity Analysis with Partial Rank Correlation Coefficients
In 1-vector models, positive correlation with R0 was found for transmission rates, biting rates, mosquito survival rates, mosquito population growth rates, mosquito maturation rates, mosquito carrying capacity, and rates of LACV dissemination among mosquitoes (Figure 4). In contrast, rates of host recovery were negatively correlated with R0, as was host population size. Although this latter result is somewhat counterintuitive, it is well known for systems with a saturating functional response (28).
In the 2-vector model, most PRCC values were reduced but maintained the same sign. This finding reflects the similar effect but lesser role of either mosquito species individually when both species are present. Not surprisingly, PRCC reductions were more severe for the tiger mosquito, which is the less competent vector in the 2-vector system. Although most PRCC values merely exhibited reductions in the 2-vector model, several underwent more striking changes. First, the tiger mosquito population growth rate and the tiger mosquito carrying capacity switched from being positively correlated with R0 (strongly so in the case of carrying capacity) in the tiger model to being negatively correlated with R0 in the tree-hole/tiger model. This switch occurs because tiger mosquitoes are generally detrimental to LACV spread in systems in which the native vector is also present, a conclusion that accords with our general finding that tiger mosquitoes should, if anything, reduce LACV transmission. Second, the population growth rate of the tree-hole mosquito actually became more strongly correlated with R0 when tiger mosquitoes were present. The explanation is as follows. In the tree-hole/tiger model, this parameter helps to influence the outcome of interspecific competition. Specifically, high tree-hole mosquito growth rates give this species an increased chance for survival against the more aggressive, generally more fecund, tiger mosquito population.
Although PRCC analyses can identify correlations between model parameters and disease outcomes, large PRCC values additionally indicate model parameters that contribute a high degree of uncertainty to model predictions. In the tree-hole/tiger model, the largest sources of uncertainty in R0 were the survival rate of tree-hole mosquitoes, the biting rate of tree-hole mosquitoes, and interspecific competition of tiger and tree-hole mosquitoes. In the tree-hole model and the tiger model, the largest contributions to uncertainty were survival and biting rates but also vector carrying capacities.
In contrast to previously published conclusions (29), our model suggests that LACV should be sustainable in 46%–60% of scenarios in which the tree-hole mosquito serves as the sole vector. This conclusion still indicates a sizeable number of scenarios in which LACV transmission should not occur. One interpretation is that LACV spread is only marginally favorable and that small changes in system characteristics (e.g., different mosquito or virus strains or environmental conditions) are sufficient to initiate or suppress disease transmission. This marginal favorability could explain the patchy detection of LACV across its native range (9) and the sudden appearance of LACV at sites where it was previously absent. The potential for variability in epidemiologic parameters raises the question of how to predict when and where LACV might emerge. Although measuring every system parameter at every local site is not feasible, our PRCC analysis suggests that careful attention to vector survival, competition, biting rates, and carrying capacities would be beneficial.
One factor that does not explain the emergence of novel LACV foci is the invasion of tiger mosquitoes. We predict that the invasive tiger mosquito should actually reduce disease transmission in wildlife reservoirs and human populations (even accounting for the fact that tiger mosquitoes are aggressive human biters). Thus, the presence of the invasive tiger mosquito does not sufficiently explain the dramatic increase in LACV disease cases in Appalachia (8,12–14,30) (http://trace.tennessee.edu/utk_gradthes/1788/), suggesting that correlations between tiger mosquito invasion and the epidemiologic risk for LACV disease are driven by other, concomitant, changes. In support of this conclusion is the absence of any increase in LACV disease prevalence in the Midwest, despite the presence of a tiger mosquito infestation. Indeed, reported cases in the region have decreased (12), consistent with predictions from our model, but may also be independent of the arrival of tiger mosquitoes (see Midwest LAC Cases, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html).
Having ruled out a straightforward contribution of invading tiger mosquitoes to LACV disease emergence, we consider the possibility that tiger mosquitoes are responsible for recent changes in LACV epidemiology. One potential mechanism involves indirect effects on the native vector. Tree-hole mosquitoes that survive competition with tiger mosquitoes are generally larger and more competent LACV vectors (31), which could increase the likelihood of LACV transmission. Another possible mechanism is niche differentiation. In general, mosquito competition is quantified by raising the larvae of competing species together in 1 container and then assessing growth metrics such as survival or maturation time. Although this approach enables estimation of direct competition, it does not capture spatial (32) or temporal niche partitioning that can decrease the strength of interspecific competition. If tiger mosquitoes do not reduce the tree-hole population as severely as our model predicts, then their dampening effect on LACV transmission will, likewise, be diminished (see Conditions Under Which Tiger Mosquitoes Enable LAC Spread at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html). Last, our estimates for LACV transmission to and from tiger mosquitoes are based on 1 study that used an LACV strain predating establishment of the tiger mosquito in the United States (17). Given that transmission studies can be highly variable and that, since introduction of the tiger mosquito into the United States, local LACV strains may have adapted to be more suitable in this new host, it is also possible that our estimates for tiger mosquito competence are overly low (see Conditions Under Which Tiger Mosquitoes Enable LAC Spread, at http://www.clfs.umd.edu/biology/faganlab/disease-ecology.html). Recent evidence finding substantial infection rates in a tiger mosquito population in Tennessee (33) supports this conclusion, although further transmission studies and analyses of virus evolution are warranted.
One final explanation for the recent emergence of LACV, and the explanation that we favor, is that our model predictions are correct and that other factors beyond tiger mosquitoes are responsible for the change. A promising contender is the Asian bush mosquito (Ochlerotatus japonicus), hereafter referred to as the bush mosquito. This mosquito is a second container-breeding invasive species that, like the tiger mosquito, seems to have arrived in North America in a shipment of tires (34). Because the bush mosquito was introduced more recently than the tiger mosquito (34), it has not been studied as extensively, particularly in the context of LACV. Nevertheless, laboratory work has demonstrated its competence as an LACV vector (35), and LACV has been isolated from field-collected pools of these mosquitoes (36). The role of bush mosquitoes in LACV transmission may be studied by using a model similar to that presented here. However, this study would require additional empirical work, including characterization of transovarial transmission by this species.
Beyond the introduction of novel vectors, other changes (e.g., climatologic variables [4], human demographics [37], wildlife densities [38], and land use [https://vtechworks.lib.vt.edu/handle/10919/64932]) may also contribute to LACV emergence. According to our PRCC analysis, for example, small changes in adult mosquito survival rates could dramatically alter R0. Mosquito survival rates not only increase the equilibrium size of mosquito populations but also increase the likelihood of mosquitoes surviving to their second or third blood meals, which is necessary for horizontal LACV transmission. Decreases in mosquito predators, varying from birds to spiders (38–41), could thus strongly affect LACV prevalence. Our PRCC analysis also indicates that mosquito carrying capacities have a substantial effect on LACV persistence. Consequently, even small increases in container availability (e.g., new tire yards or unemptied backyard planters) should have dramatic effects on LACV disease incidence rates. Last, substantial growth has occurred in southern Appalachia over the past 30 years (37); thus, even without increased enzootic transmission, absolute cases may have increased from population growth alone. Although purely speculative, such habitat and demographic changes may be the true cause of the recent emergence of LACV.
That tiger mosquito invasion is not predicted to increase LACV transmission or even human cases highlights an important issue at the interface between disease ecology and invasion biology. In particular, this finding shows that predicting whether an invasive vector will exacerbate or dampen the spread of a disease can be complex and can depend on an elaborate network of species interactions. Although this network includes obvious disease interactions like horizontal and vertical transmission, it also includes ecologic interactions that may be relatively independent of the disease itself. In the LACV system, for example, competition between native tree-hole mosquitoes and invasive tiger mosquitoes strongly influences whether or not LACV persists (Figure 4). Indeed, as a consequence of this competition, tiger mosquitoes can drive local extinction of LACV, despite the fact that tiger mosquitoes can acquire and transmit the virus, making them seem to be competent vectors.
Because of the complexity of disease transmission in ecologic systems, it is often hard to identify the causes of altered disease epidemiology. However, faced with increasing climate and landscape change, ongoing introduction of novel invasive species (pathogens and vectors), and emerging or reemerging diseases, an understanding of the effects of these different forms of global change on disease dynamics is essential. We have moved toward this goal by developing a framework for investigating the role of invasive vectors on the transmission of a native disease. Using LACV as an example, our model highlights the fact that the introduction of a new disease vector does not guarantee increased disease transmission and, in fact, can even drive local extinction of an endemic pathogen.
Dr. Bewick is a research scientist in the Department of Biology at the University of Maryland, College Park. She is interested in vectorborne diseases and how they are affected by insect life history and community ecology.
Acknowledgment
This research was supported by the US National Science Foundation under grant DMS-1225917 (to W.F.F.) and by the Strategic Environmental Research and Development Program under grant RC-2639 (to S.B.).
References
- Christofferson RC. Arbovirus phenotype alters transmission potential. Baton Rouge (LA): Louisiana State University; 2011.
- Egger JR, Ooi EE, Kelly DW, Woolhouse ME, Davies CR, Coleman PG. Reconstructing historical changes in the force of infection of dengue fever in Singapore: implications for surveillance and control. Bull World Health Organ. 2008;86:187–96.DOIPubMedGoogle Scholar
- Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–83.DOIPubMedGoogle Scholar
- Parham PE, Waldock J, Christophides GK, Hemming D, Agusto F, Evans KJ, Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Philos Trans R Soc Lond B Biol Sci. 2015;370:20130551. DOIPubMedGoogle Scholar
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85.DOIPubMedGoogle Scholar
- Rust RS, Thompson WH, Matthews CG, Beaty BJ, Chun RW. La Crosse and other forms of California encephalitis. J Child Neurol. 1999;14:1–14.DOIPubMedGoogle Scholar
- Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev. 1994;7:89–116.DOIPubMedGoogle Scholar
- Jones TF, Craig AS, Nasci RS, Patterson LE, Erwin PC, Gerhardt RR, Newly recognized focus of La Crosse encephalitis in Tennessee. Clin Infect Dis. 1999;28:93–7.DOIPubMedGoogle Scholar
- Haddow AD, Odoi A. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003-2007. PLoS One. 2009;4:e6145.DOIPubMedGoogle Scholar
- Grimstad P. California group virus disease. In: Monath TP, editor. The arboviruses: epidemiology and ecology. Vol. 2. Boca Raton (FL): CRC Press. 1988. p. 99–136.
- Moulton DW, Thompson WH. California group virus infections in small, forest-dwelling mammals of Wisconsin. Some ecological considerations. Am J Trop Med Hyg. 1971;20:474–82.PubMedGoogle Scholar
- Leisnham PT, Juliano SA. Impacts of climate, land use, and biological invasion on the ecology of immature Aedes mosquitoes: implications for La Crosse emergence. Ecohealth. 2012;9:217–28.DOIPubMedGoogle Scholar
- McJunkin JE, de los Reyes EC, Irazuzta JE, Caceres MJ, Khan RR, Minnich LL, La Crosse encephalitis in children. N Engl J Med. 2001;344:801–7.DOIPubMedGoogle Scholar
- Nasci RS, Moore CG, Biggerstaff BJ, Panella NA, Liu HQ, Karabatsos N, La Crosse encephalitis virus habitat associations in Nicholas County, West Virginia. J Med Entomol. 2000;37:559–70.DOIPubMedGoogle Scholar
- Sprenger D, Wuithiranyagool T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J Am Mosq Control Assoc. 1986;2:217–9.PubMedGoogle Scholar
- Cully JF Jr, Streit TG, Heard PB. Transmission of La Crosse virus by four strains of Aedes albopictus to and from the eastern chipmunk (Tamias striatus). J Am Mosq Control Assoc. 1992;8:237–40.PubMedGoogle Scholar
- Grimstad PR, Kobayashi JF, Zhang MB, Craig GB Jr. Recently introduced Aedes albopictus in the United States: potential vector of La Crosse virus (Bunyaviridae: California serogroup). J Am Mosq Control Assoc. 1989;5:422–7.PubMedGoogle Scholar
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001;7:807–11.DOIPubMedGoogle Scholar
- Westby K, Fritzen C, Huang J, Jaske E, Paulsen D, Jones C, La Crosse encephalitis in eastern Tennessee: evidence of invasive mosquito (Aedes albopictus and Ochlerotatus japonicus) involvement in the transmission of an indigenous disease. Am J Trop Med Hyg. 2011;85(suppl):374.PubMedGoogle Scholar
- Matser A, Hartemink N, Heesterbeek H, Galvani A, Davis S. Elasticity analysis in epidemiology: an application to tick-borne infections. Ecol Lett. 2009;12:1298–305.DOIPubMedGoogle Scholar
- Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. International Statistical Review/Revue Internationale de Statistique. The Hague (the Netherlands): International Statistical Institute. 1994. p. 229–43.
- Gauld LW, Hanson RP, Thompson WH, Sinha SK. Observations on a natural cycle of La Crosse virus (California group) in Southwestern Wisconsin. Am J Trop Med Hyg. 1974;23:983–92.PubMedGoogle Scholar
- Barker CM, Paulson SL, Cantrell S, Davis BS. Habitat preferences and phenology of Ochlerotatus triseriatus and Aedes albopictus (Diptera: Culicidae) in southwestern Virginia. J Med Entomol. 2003;40:403–10.DOIPubMedGoogle Scholar
- Szumlas DE, Apperson CS, Powell EE, Hartig P, Francy DB, Karabotsos N. Relative abundance and species composition of mosquito populations (Diptera:Culicidae) in a La Crosse virus-endemic area in western North Carolina. J Med Entomol. 1996;33:598–607.DOIPubMedGoogle Scholar
- Kappus KD, Calisher CH, Baron RC, Davenport J, Francy DB, Williams RM. La Crosse virus infection and disease in western North Carolina. Am J Trop Med Hyg. 1982;31:556–60.PubMedGoogle Scholar
- Beaty BJ, Thompson WH. Emergence of La Crosse virus from endemic foci. Fluorescent antibody studies of overwintered Aedes triseriatus. Am J Trop Med Hyg. 1975;24:685–91.PubMedGoogle Scholar
- Lisitza M, DeFoliart G, Yuill T, Karandinos M. Prevalence rates of LaCrosse virus (California encephalitis group) in larvae from overwintered eggs of Aedes triseriatus. Mosq News. 1977;37:745–50.
- Wonham MJ, Lewis MA, Rencławowicz J, van den Driessche P. Transmission assumptions generate conflicting predictions in host-vector disease models: a case study in West Nile virus. Ecol Lett. 2006;9:706–25.DOIPubMedGoogle Scholar
- DeFoliart GR. Aedes triseriatus: vector biology in relationship to the persistence of La Crosse virus in endemic foci. Prog Clin Biol Res. 1983;123:89–104.PubMedGoogle Scholar
- Erwin PC, Jones TF, Gerhardt RR, Halford SK, Smith AB, Patterson LE, La Crosse encephalitis in Eastern Tennessee: clinical, environmental, and entomological characteristics from a blinded cohort study. Am J Epidemiol. 2002;155:1060–5.DOIPubMedGoogle Scholar
- Bevins SN. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae). Biol Invasions. 2008;10:1109–17 .DOIGoogle Scholar
- Lounibos L, O’meara G, Escher R, Nishimura N, Cutwa M, Nelson T, Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Invasions. 2001;3:151–66 .DOIGoogle Scholar
- Westby KM, Fritzen C, Paulsen D, Poindexter S, Moncayo AC. La Crosse encephalitis virus infection in field-collected Aedes albopictus, Aedes japonicus, and Aedes triseriatus in Tennessee. J Am Mosq Control Assoc. 2015;31:233–41.DOIPubMedGoogle Scholar
- Kampen H, Werner D. Out of the bush: the Asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasit Vectors. 2014;7:59.DOIPubMedGoogle Scholar
- Sardelis MR, Turell MJ, Andre RG. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae). J Med Entomol. 2002;39:635–9.DOIPubMedGoogle Scholar
- Harris MC, Dotseth EJ, Jackson BT, Zink SD, Marek PE, Kramer LD, La Crosse Virus in Aedes japonicus japonicus mosquitoes in the Appalachian region, United States. Emerg Infect Dis. 2015;21:646–9.DOIPubMedGoogle Scholar
- Pollard KM. Population growth and distribution in Appalachia: new realities. Washington (DC): Appalachian Regional Commission; 2005.
- Hartman K, Roles E. Animal Diversity Web. Aedes albopictus [cited 2016 Sep 14]. http://animaldiversity.org/accounts/Aedes_albopictus/
- Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, Bat white-nose syndrome: an emerging fungal pathogen? Science. 2009;323:227.DOIPubMedGoogle Scholar
- Reiskind MH, Wund MA. Experimental assessment of the impacts of northern long-eared bats on ovipositing Culex (Diptera: Culicidae) mosquitoes. J Med Entomol. 2009;46:1037–44.DOIPubMedGoogle Scholar
- Rund SS, O’Donnell AJ, Gentile JE, Reece SE. Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects. 2016;7:14.DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 22, Number 11—November 2016
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Sharon Bewick, University of Maryland, 1210 Biology-Psychology Building, College Park, MD 20742, USA
Top