Volume 22, Number 3—March 2016
Research
Decreased Time to Treatment Initiation for Multidrug-Resistant Tuberculosis Patients after Use of Xpert MTB/RIF Test, Latvia
Figure 4
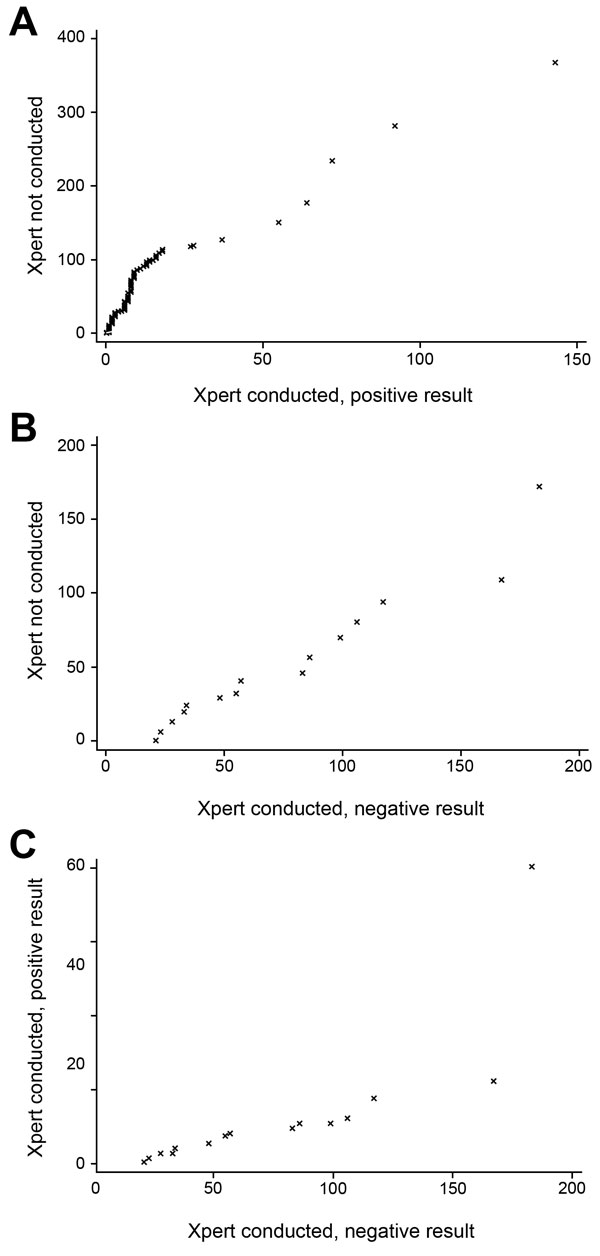
Figure 4. Quantile–quantile plots of time to multidrug-resistant tuberculosis (MDR TB) treatment initiation by use and results of Xpert MTB/RIF (Xpert) for patients with MDR TB, Latvia, 2009–2012. Shown are time to MDR TB treatment initiation (days) for patients A) who were not tested by Xpert vs. those who had rifampin-resistant TB by Xpert, B) those who were not tested vs. those who had a negative result for rifampin-resistant TB, and C) those who were tested by Xpert and had positive vs. negative results for rifampin-resistant TB. MTB, Mycobacterium tuberculosis; RIF, rifampin.
1These authors contributed equally to this article.
Page created: March 01, 2016
Page updated: March 01, 2016
Page reviewed: March 01, 2016
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.