Volume 22, Number 4—April 2016
Research
Quantifying Transmission of Clostridium difficile within and outside Healthcare Settings
Abstract
To quantify the effect of hospital and community-based transmission and control measures on Clostridium difficile infection (CDI), we constructed a transmission model within and between hospital, community, and long-term care-facility settings. By parameterizing the model from national databases and calibrating it to C. difficile prevalence and CDI incidence, we found that hospitalized patients with CDI transmit C. difficile at a rate 15 (95% CI 7.2–32) times that of asymptomatic patients. Long-term care facility residents transmit at a rate of 27% (95% CI 13%–51%) that of hospitalized patients, and persons in the community at a rate of 0.1% (95% CI 0.062%–0.2%) that of hospitalized patients. Despite lower transmission rates for asymptomatic carriers and community sources, these transmission routes have a substantial effect on hospital-onset CDI because of the larger reservoir of hospitalized carriers and persons in the community. Asymptomatic carriers and community sources should be accounted for when designing and evaluating control interventions.
Infection with the nosocomial pathogen Clostridium difficile is a major risk in healthcare settings and long-term care facilities (LTCFs) and has an increasing prevalence in the broader community. Infection is diagnosed in >250,000 hospitalized persons annually in the United States (1). Colonization of the gut microbiota with C. difficile can be innocuous and asymptomatic. However, antimicrobial drugs disrupt the normal intestinal microbial architecture and can enable proliferation of C. difficile (2). An insufficient host antibody response to C. difficile toxins A and B can then lead to C. difficile infection (CDI). CDI is a severe diarrheal disease that is concentrated among elderly persons and those with extended hospital stays or residing in LTCFs. The relative risk for CDI, given recent antimicrobial drug exposure, differs greatly among antimicrobial drug classes and ranges from no relative risk when receiving tetracyclines to a 20-fold relative risk when receiving clindamycin (2). Despite an increasing interest in C. difficile biology and the epidemiology of CDI, fundamental questions about reservoirs and routes of transmission remain unanswered.
Molecular typing and contact tracing studies have estimated that 10%–38% of CDI cases that occur >48 hours after hospital admission (termed hospital-onset CDI) can be attributed to transmission from known symptomatic contacts within the hospital (3–6). These estimates suggest that a substantial proportion of CDI arises from other sources, such as transmission from patients with asymptomatic colonization or community acquisition (3,5,7,8). The relative role of these routes of transmission to the epidemiology of C. difficile is crucial for determining effectiveness of hospital-based measures to control infection. In addition, toxin-targeting treatments, such as vaccines, nontoxigenic C. difficile, and monoclonal antibodies, might protect against CDI but are unlikely to prevent asymptomatic colonization with C. difficile (9). To predict the effectiveness of these emerging therapies, it is critical to understand the role of asymptomatic carriers in CDI epidemiology.
Mathematical models of C. difficile colonization have generated insights regarding the epidemiologic role of antimicrobial drugs on CDI outbreaks (10). Such models have also quantified the effect of hospital-based control interventions (11–14) and demonstrated the crucial roles of asymptomatic colonization and patients with exposure before hospital admission in sustaining hospital transmission (7,13). Most studies have focused on the hospital setting. To fully understand the epidemiology of the pathogen and to inform decisions regarding control strategies, it is crucial to quantify the relative transmission of C. difficile in the hospital and in the broader community (8).
To evaluate the relative role of asymptomatic hospital transmission, symptomatic hospital transmission, LTCF transmission, and community transmission, we integrated diverse clinical and epidemiologic data into a dynamic model of C. difficile transmission within and among hospitals, LTCFs, and community settings in the United States. We parameterized our model by using Medicare and Healthcare Cost and Utilization Project databases and data from published epidemiologic and clinical research. To estimate infectivity of symptomatic and asymptomatic patients in the hospital; corresponding infectivity of persons in LTCFs and in the community; and average risks for acquiring C. difficile in the hospital, LTCF, and the community, we fit our model to estimated toxigenic C. difficile colonization and CDI incidence in each of these settings. Furthermore, we calculated the effect on CDI incidence of targeting key aspects of CDI epidemiology with control interventions in each of the 3 settings.
Definitions
We refer to acquisition of C. difficile from human sources as C. difficile transmission and acquisition of C. difficile from nonhuman sources as nonhuman acquisition. Asymptomatic persons carrying C. difficile are referred to as colonized. Persons carrying C. difficile and symptomatic for diarrheal disease associated with C. difficile are referred to as persons with CDI.
Model Structure
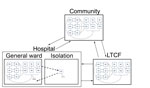
Previous models have focused almost exclusively on the hospital setting (7,8,10,12). We constructed a new model that encompasses C. difficile transmission and symptomatic CDI within a hospital, an LTCF, and an associated mid-sized community and quantifies patient movement between these settings. We parameterized our model with data from a combination of sources, including published literature, the US Census, national hospital and LTCF surveys, and the Healthcare Cost and Utilization Project and Medicare databases (Technical Appendix).
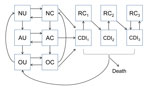
We structured our model in compartments (Figure 1) composed of patients who are currently receiving antimicrobial drugs, those who have a history of antimicrobial drug use and an increased risk for CDI, or those who do not have a recent history of receiving antimicrobial drugs. Consistent with clinical observations (15), we assumed that the increased risk for CDI after antimicrobial drug use reverted to normal in an average of 45 days. Uncolonized patients could become asymptomatically colonized with C. difficile because of transmission from asymptomatic patients, transmission from patients with CDI, or through acquisition from background sources in the community. Asymptomatically colonized patients could remain asymptomatic, spontaneously clear their colonization, or develop symptomatic CDI. Patients with CDI could recover and be at temporarily increased risk for recolonization, could recover and remain colonized and at risk for recurrence, or could die from the disease. We included 3 CDI and recurrence classes, each with a successively higher likelihood of recurrence, to reflect clinical observations of the increasing likelihood of recurrence after multiple CDI episodes (16–18). We assumed that all patients with CDI were first asymptomatically colonized before symptoms developed.
We embedded this epidemiologic model within a model of patient flow between the hospital, LTCF, and community (Figure 2), parameterized from national hospital and long-term-care-facility survey data. Patients with CDI remained hospitalized for an additional 3.1 days (95% CI 2.3–4.0 days) (19–21). Patients with CDI had a 96% (95% CI 93%–99%) probability of being given a diagnosis and subjected to isolation protocols that reduced transmission by 53% (95% CI 37%–72%) (22–25). We further assumed that persons in the community and in an LTCF in whom CDI developed were hospitalized with probabilities of 26% (95% CI 23%–28%) and 27% (95% CI 23%–32%), respectively (Table 1) (26,27).
Demographics
To represent demographically stratified CDI risk between the 3 settings, we modeled 5 demographic groups: persons <50 years of age, those 50–65 years of age without concurrent conditions, those 50–65 years of age with concurrent conditions, those >65 years of age without concurrent conditions, and those >65 years of age with concurrent conditions. Therefore, our full model consisted of base epidemiology (Figure 1) applied to each of the 5 demographic groups, and each group populated and moved between the hospital, LTCF, and the community (Figure 2) at rates calibrated from published C. difficile literature, US hospital discharge and census data, and Medicare and Healthcare Cost and Utilization Project databases (Technical Appendix Table 4). We assumed that colonized patients with concurrent conditions are at greater risk for development of CDI (online Technical Appendix).
Transmission
We specified 5 C. difficile transmission rates: 1) the base CDI rate at which patients without a diagnosis and symptomatic CDI transmit in the hospital, 2) the base asymptomatic rate at which asymptomatically colonized patients transmit in the hospital, 3) the LTCF transmission rate representing the relative infectivity of persons in LTCFs compared with patients in the hospital, 4) the community transmission rate representing the relative infectivity of persons in the community compared with patients in the hospital, and 5) the rate of C. difficile acquisition from nonhuman reservoirs. We further defined the force of colonization as the rate at which uncolonized patients become asymptomatically colonized with C. difficile and specified 3 separate force-of-colonization rates: 1) the hospital, 2) LTCF, and 3) the community.
For the force of colonization in the hospital, we specified that nonisolated symptomatic patients with CDI transmit at the base CDI rate, that isolated patients with CDI transmit at the base CDI rate multiplied by the probability that isolation measures are insufficient, and that asymptomatically colonized patients transmit at the base asymptomatic rate. We assumed direct contact mixing and density-dependent transmission, which is consistent with the observation that larger hospitals have greater CDI incidence than smaller hospitals (36). Environmental contamination and transmission mediated by healthcare workers were implicitly included by our calibration of the base CDI rate and the base asymptomatic rate. Hospital hygiene was separated into 2 components: overall hospital hygiene, which influenced transmission from asymptomatically colonized patients and from undiagnosed patients with CDI; and the probability of, and effectiveness of, enhanced isolation protocols for patients given a diagnosis of CDI.
For the force of colonization in the LTCF, we made 3 assumptions. First, enhanced isolation protocols were not available. Second, patients with CDI transmit at the base CDI rate multiplied by the LTCF transmission rate modifier. Third, asymptomatically colonized patients transmit at the base asymptomatic rate multiplied by the LTCF transmission rate modifier.
For the force of colonization in the community, we assumed that C. difficile could be acquired from nonhuman reservoirs (37), that patients with CDI transmit at the base CDI rate multiplied by the community transmission rate modifier, and that asymptomatically colonized patients transmit at the base asymptomatic rate multiplied by the community transmission rate modifier. Because there are insufficient published data with which to statistically differentiate between human transmission in the community and nonhuman acquisition, we estimated the force of colonization directly during our model calibration and then calculated the upper bounds for the community transmission rate modifier and for the rate of nonhuman acquisition.
Although age, history of antimicrobial drug use, and concurrent conditions are predictors of diarrheal CDI, they are not predictors of asymptomatic C. difficile colonization (38,39). Therefore, we assumed that the rate at which symptomatic CDI developed in colonized patients was dependent on age, antimicrobial drug use, concurrent conditions, and hospitalization status. Transmission parameters and force of colonization were independent of age, antimicrobial drug use or concurrent conditions (online Technical Appendix).
Calibration
We used the Markov Chain Monte Carlo Metropolis algorithm (40) to calibrate our stochastic model and combined prior parameter densities (Table 1) with epidemiologic data, including asymptomatic prevalence and CDI incidence in the hospital, LTCF, and community Technical Appendix Table 2). This analysis yielded an ensemble of 1,000 parameter sets that estimated the joint posterior distribution for parameters with prior literature estimates (Table 1) for the 5 transmission parameters and for the base rate at which CDI developed in asymptomatically colonized persons (Table 2). Details of coding, the stochastic model, and calibration are provided in the online Technical Appendix.
Epidemiologic Analysis
To estimate relative infectivity of a hospitalized patient with CDI compared with a hospitalized asymptomatically colonized patient, accounting for isolation protocols, we computed the ratio of 1) the base CDI transmission rate from a hospitalized patient with CDI multiplied by the probability that the patient is either not given a diagnosis or that isolation protocols are improperly implemented to 2) the base asymptomatic transmission rate from a hospitalized, asymptomatically colonized patient. To generate a posterior distribution for this ratio, we repeated this calculation for each of the 1,000 runs in our posterior sample. To estimate the average risk for a person to become exposed to and colonized with C. difficile, for each of the runs, we computed the average force of colonization within the hospital, community, and LTCF.
To estimate an upper bound for the community transmission rate and for nonhuman acquisition, we first computed the daily average community force of colonization, which represents the sum of C. difficile transmission from other persons in the community plus acquisition from nonhuman reservoirs. By setting the nonhuman acquisition rate to 0, we calculated an upper bound for the community transmission rate. Likewise, by setting the community transmission rate to 0, we calculated an upper bound for nonhuman acquisition. We repeated this step for each of the 1,000 runs and generated posterior distributions for the upper bounds of the community transmission rate and the nonhuman acquisition rate.
Control Strategy Analysis
To quantify the effect of transmission control interventions on CDI incidence, we varied each of the following factors: CDI diagnosis rate of a hospitalized patient with CDI, effectiveness of isolation protocols for a patient given a diagnosis, overall hospital hygiene, improvements in community transmission, and improvements in LTCF transmission across a range from 0 to double the model-fitted maximum likelihood estimate and while sampling all other model parameters from their posterior distributions. We used linear regression to determine the reduction for hospital-onset CDI, community-onset CDI, and LTCF-onset CDI incidence per 1% improvement in each transmission control intervention.
To compute the effect of different classes of antimicrobial drugs on CDI incidence, we varied the antimicrobial drug risk ratio in the hospital from 1, which is representative of low-risk antimicrobial drugs (e.g., tetracyclines), to 20, which is representative of high-risk antimicrobial drugs (e.g., clindamycin) (2). While varying the antimicrobial drug risk ratio, we sampled all other parameters, including community and LTCF antimicrobial drug risk, from their posterior distributions, thereby obtaining 95% CIs for our estimates of the effect of antimicrobial drug class on CDI incidence. We repeated this analysis for antimicrobial drug risk in the community and the LTCF. We then calculated changes in hospital-onset CDI, community-onset CDI, and LTCF CDI incidence as hospital, community, and LTCF risk for antimicrobial drug use were varied.
Epidemiology
For within the hospital, we computed that the ratio of transmission from an isolated symptomatic patient with CDI with transmission from an asymptomatic patient was 15 (95% CI 7.2–32) (Table 2). This high ratio indicates that a symptomatic patient with CDI contributes more to transmission than does an asymptomatically colonized patient, even after accounting for C. difficile protocols. Within the LTCF, the transmission rate from a person with CDI to an uncolonized person is 27% (95% CI 13%–51%) that of the hospital, and the transmission rate from an asymptomatically colonized person to an uncolonized person is 13% (95% CI 6.8%–22%) that of the hospital. Within the community, the transmission rate from a person with CDI to an uncolonized person is 0.1% (95% CI 0.062%–0.2%) that of the hospital, and the transmission rate from an asymptomatically colonized person to an uncolonized person is 0.052% (95% CI 0.033%–0.089%) that of the hospital (Table 2).
To estimate the average risk for a person to become exposed to and be colonized with C. difficile, we computed the force of colonization. We calculated that an uncolonized person in the hospital has a probability of 2.3% (95% CI 1.7%–3.2%) per day of acquiring C. difficile and becoming a carrier (with or without symptoms); an uncolonized person in the community has a probability of 0.12% (95% CI 0.050%–0.23%) per day, and a person in an LTCF has a probability of 0.37% (95% CI 0.096%–0.77%) per day (Table 2). These results provide a quantitative estimate of the average risk for C. difficile exposure to persons in each setting.
Control Strategy
To estimate the effect of transmission control interventions on CDI incidence, we computed the percentage reduction in hospital-onset CDI, community-onset CDI, and LTCF CDI per percentage improvement in hospital CDI diagnosis rate, effectiveness of isolation protocols, overall hospital hygiene, transmission in the community, and transmission in an LTCF (Figure 3). We found that CDI diagnosis rate, effectiveness of isolation, overall hospital hygiene, and transmission in the community, but not transmission in an LTCF, affected hospital-onset CDI. In addition, community-onset CDI and LTCF CDI were not affected by hospital-based transmission interventions.
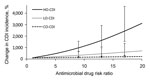
As the relative risk for antimicrobial drug class prescribed within each of the settings was increased, the CDI incidence likewise increased within that setting (Figure 4). However, there was no relationship between the antimicrobial drug class prescribed within a location and CDI incidence in another location. Specifically, we estimated that for every unit increase in antimicrobial drug risk ratio, the CDI incidence increased by 160% (95% CI 98%–320%) in the hospital, 33% (95% CI 13%–83%) in the LTCF, and 6.4% (95% CI 3.9%–13%) in the community. These results indicate that the effect of antimicrobial drug risk on CDI incidence is intertwined with C. difficile transmission dynamics, which differ between the hospital, LTCF, and community.
Through stochastic simulation and Bayesian model calibration, we estimated C. difficile transmission rates within and outside the healthcare setting. We also quantified the effect on CDI incidence of control interventions that reduce these transmission rates. We found that a person with CDI in an LTCF transmits at a rate 27% that for a comparable patient in the hospital, and a colonized person or a person with CDI in the community transmits C. difficile to others at a rate <0.1% that of a comparable patient in the hospital. Despite the lower community transmission rate, we found that because of the much larger pool of colonized persons in the community, interventions that reduce community transmission hold substantial potential to reduce hospital-onset CDI by reducing the number of patients entering the hospital with asymptomatic colonization. Moreover, our results show that in the hospital, symptomatic CDI patients under isolation and infection control measures nonetheless transmit CDI to uncolonized patients at a rate that is 15 times greater than that of asymptomatic carriers. This higher rate of transmission indicates that toxin-targeting treatments (such as vaccines); nontoxigenic C. difficile; and monoclonal antibodies, which might protect against symptomatic CDI but not against asymptomatic colonization, could be effective tools for reducing not only primary CDI cases but also for further transmission (9).
Our epidemiologic results underscore the need for incorporating and understanding transmission dynamics within and outside healthcare settings when evaluating C. difficile control strategies. Although C. difficile transmission rates are lower among asymptomatically colonized persons, residents of LTCFs, and persons in the community than in hospitalized patients with symptomatic CDI, overall CDI incidence is driven by several factors: transmission, antimicrobial drug use, and underlying population health. We found that, per unit increase in relative antimicrobial drug risk, CDI incidence increases by a factor of 160% in the hospital and 33% in the LTCF but only by a factor of 6.4% in the community. This finding is a consequence of amplification by concentration.
When we compared patients in the hospital and LTCF with persons in the community, we found that patients are closer to each other, are more frequently receiving antimicrobial drugs, and tend to have poorer overall health or may be immunocompromised. These attributes combine to yield a greater risk for infection and transmission. This finding of amplification-by-concentration has major implications for antimicrobial drug risk management: those antimicrobial drugs strongly associated with CDI, such as clindamycin, cephalosporins, and fluoroquinolones (2), will have a more detrimental effect on overall CDI incidence in a high-transmission setting, such as a hospital, than they will in a moderate-transmission setting, such as an LTCF, or in a low-transmission setting, such as the community.
We found no major effect of hospital-based transmission interventions on LTCF-onset CDI or of LTCF-based transmission interventions on hospital-onset CDI. This finding suggests that although C. difficile can be introduced by a patient who acquired the bacteria in the hospital, CDI outbreaks in LTCFs are driven primarily from within and are best mitigated by targeted transmission interventions within the facility. Likewise, any interventions to reduce transmission within an LTCF will have limited effect on hospital-onset CDI because LTCF transmission interventions will not influence continued introduction of C. difficile to the hospital from the community.
The control strategies we evaluated (Figure 3) are representative of a broad range of interventions. For example, an improvement in hospital isolation effectiveness could be achieved through enhanced hospital staff adherence to precautions, or alternatively through an increased capacity to keep a patient with CDI in isolation for the duration of the disease. An improvement in the LTCF transmission rate could be achieved through an improvement to LTCF staff hygiene and cleanliness, through an increased availability of private facilities for residents, or through the isolation of LTCF residents with CDI.
Although there are few data with which to differentiate the sources of community-associated C. difficile, we were able to use a community C. difficile colonization study (37) to calibrate our model. From our calibrated model, we estimated the overall community force of colonization and calculated an upper bound for the community transmission rate. Future studies of similar design but with greater statistical power than the study used for our calibration (37), which survey healthy, nonhospitalized adults for asymptomatic C. difficile carriage while differentiating community risk factors, would provide the necessary data with which our model could directly quantify transmission from human sources and acquisition from nonhuman reservoirs.
Our analyses demonstrated that C. difficile transmission among healthcare settings and the community is interconnected, and there are comparable effects of community-based transmission and hospital-based transmission on hospital-onset CDI. We found that the effect of antimicrobial drug use on CDI incidence is modulated by transmission dynamics, with specific antimicrobial drugs exacerbating incidence, and doing so to a greater degree in high-transmission settings than in low-transmission settings. These results underscore the need for empirical quantification of community-associated transmission and the need of understanding transmission dynamics in all settings when evaluating C. difficile interventions and control strategies.
A.P.G., J.P.T., and D.P.D. have received research support from Sanofi-Pasteur and have consulted for Sanofi-Pasteur and Merck. M.A.O. has received research support from Sanofi-Pasteur and Pfizer and has been a consultant for Sanofi-Pasteur, Pfizer, and Merck. E.R.D. has received research support from Rebiotix, Microdermis, Merck, and Sanofi-Pasteur and has been a consultant for Sanofi-Pasteur, Rebiotix, Pfizer, Valneva, Merck, Summitt, and Daiichi.
Dr. Durham is an associate research scientist in the Center for Infectious Disease Modeling and Analysis at the Yale School of Public Health, New Haven, Connecticut. His research focuses on using mathematical and computational models to quantify the impact of control interventions upon Clostridium difficile, hepatitis C virus, and human papillomavirus.
Acknowledgments
We thank Clifford McDonald for helpful comments and suggestions and Dustin Stwalley for assistance with data acquisition.
This study was supported by Sanofi-Pasteur and the Notsew Orm Sands Foundation. Sanofi Pasteur assisted with study design and data acquisition and provided feedback on the completed manuscript. Simulations were run on the Yale University Biomedical High Performance Computing Center, which is supported by National Institutes of Health grants RR19895 and RR029676-01.
References
- Zilberberg MD. Increase in adult Clostridium difficile–related hospitalizations and case-fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14:929–31. DOIPubMedGoogle Scholar
- Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Sferra TJ, Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68:1951–61 . DOIPubMedGoogle Scholar
- Svenungsson B, Burman LG, Jalakas-Pornull K, Lagergren A, Struwe J, Akerlund T. Epidemiology and molecular characterization of Clostridium difficile strains from patients with diarrhea: low disease incidence and evidence of limited cross-infection in a Swedish teaching hospital. J Clin Microbiol. 2003;41:4031–7. DOIPubMedGoogle Scholar
- Walker AS, Eyre DW, Wyllie DH, Dingle KE, Harding RM, O’Connor L, Characterisation of Clostridium difficile hospital ward–based transmission using extensive epidemiological data and molecular typing. PLoS Med. 2012;9:e1001172. DOIPubMedGoogle Scholar
- Norén T, Akerlund T, Bäck E, Sjöberg L, Persson I, Alriksson I, Molecular epidemiology of hospital-associated and community-acquired Clostridium difficile infection in a Swedish county. J Clin Microbiol. 2004;42:3635–43. DOIPubMedGoogle Scholar
- Curry SR, Muto CA, Schlackman JL, Pasculle AW, Shutt KA, Marsh JW, Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin Infect Dis. 2013;57:1094–102. DOIPubMedGoogle Scholar
- Lanzas C, Dubberke ER, Lu Z, Reske KA, Gröhn YT. Epidemiological model for Clostridium difficile transmission in healthcare settings. Infect Control Hosp Epidemiol. 2011;32:553–61. DOIPubMedGoogle Scholar
- Otten AM, Reid-Smith RJ, Fazil A, Weese JS. Disease transmission model for community-associated Clostridium difficile infection. Epidemiol Infect. 2010;138:907–14. DOIPubMedGoogle Scholar
- Gerding DN, Johnson S. Management of Clostridium difficile infection: thinking inside and outside the box. Clin Infect Dis. 2010;51:1306–13. DOIPubMedGoogle Scholar
- Starr JM, Rogers TR, Impallomeni M. Hospital-acquired Clostridium difficile diarrhoea and herd immunity. Lancet. 1997;349:426–8. DOIPubMedGoogle Scholar
- Lofgren ET, Moehring RW, Anderson DJ, Weber DJ, Fefferman NH. A mathematical model to evaluate the routine use of fecal microbiota transplantation to prevent incident and recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2014;35:18–27. DOIPubMedGoogle Scholar
- Starr JM, Campbell A, Renshaw E, Poxton IR, Gibson GJ. Spatio-temporal stochastic modelling of Clostridium difficile. J Hosp Infect. 2009;71:49–56. DOIPubMedGoogle Scholar
- Yakob L, Riley TV, Paterson DL, Clements AC. Clostridium difficile exposure as an insidious source of infection in healthcare settings: an epidemiological model. BMC Infect Dis. 2013;13:376. DOIPubMedGoogle Scholar
- Rubin MA, Jones M, Leecaster M, Khader K, Ray W, Huttner A, A simulation-based assessment of strategies to control Clostridium difficile transmission and infection. PLoS ONE. 2013;8:e80671. DOIPubMedGoogle Scholar
- Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ. 2008;179:767–72. DOIPubMedGoogle Scholar
- Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205 . DOIPubMedGoogle Scholar
- Figueroa I, Johnson S, Sambol SP, Goldstein EJC, Citron DM, Gerding DN. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S104–9. DOIPubMedGoogle Scholar
- McFarland LV. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–8. DOIPubMedGoogle Scholar
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346–53. DOIPubMedGoogle Scholar
- Dubberke ER, Butler AM, Reske KA, Agniel D, Olsen MA, D’Angelo G, Attributable outcomes of endemic Clostridium difficile–associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–8. DOIPubMedGoogle Scholar
- O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile–associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–27. DOIPubMedGoogle Scholar
- Jarvis WR, Schlosser J, Jarvis AA, Chinn RY. National point prevalence of Clostridium difficile in US health care facility inpatients, 2008. Am J Infect Control. 2009;37:263–70. DOIPubMedGoogle Scholar
- Harris AD, Pineles L, Belton B, Johnson JK, Shardell M, Loeb M, Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310:1571–80 .PubMedGoogle Scholar
- Wilkinson K, Gravel D, Taylor G, McGeer A, Simor A, Suh K, Infection prevention and control practices related to Clostridium difficile infection in Canadian acute and long-term care institutions. Am J Infect Control. 2011;39:177–82. DOIPubMedGoogle Scholar
- Sloan LM, Duresko BJ, Gustafson DR, Rosenblatt JE. Comparison of real-time PCR for detection of the tcdC gene with four toxin immunoassays and culture in diagnosis of Clostridium difficile infection. J Clin Microbiol. 2008;46:1996–2001. DOIPubMedGoogle Scholar
- Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359–67. DOIPubMedGoogle Scholar
- Pawar D, Tsay R, Nelson DS, Elumalai MK, Lessa FC, Clifford McDonald L, Burden of Clostridium difficile infection in long-term care facilities in Monroe County, New York. Infect Control Hosp Epidemiol. 2012;33:1107–12. DOIPubMedGoogle Scholar
- Hensgens MP, Goorhuis A, Dekkers OM, van Benthem BH, Kuijper EJ. All-cause and disease-specific mortality in hospitalized patients with Clostridium difficile infection: a multicenter cohort study. Clin Infect Dis. 2013;56:1108–16. DOIPubMedGoogle Scholar
- Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis. 2011;53:1100–10. DOIPubMedGoogle Scholar
- McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–75. DOIPubMedGoogle Scholar
- Dubberke ER, Reske KA, Olsen MA, McMullen KM, Mayfield JL, McDonald LC, Evaluation of Clostridium difficile–associated disease pressure as a risk factor for C. difficile-associated disease. Arch Intern Med. 2007;167:1092–7. DOIPubMedGoogle Scholar
- Simor AE, Yake SL, Tsimidis K. Infection due to Clostridium difficile among elderly residents of a long-term-care facility. Clin Infect Dis. 1993;17:672–8. DOIPubMedGoogle Scholar
- Zhang Y, Steinman MA, Kaplan CM. Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med. 2012;172:1465–71. DOIPubMedGoogle Scholar
- Hicks LA, Taylor TH, Hunkler RJ. Outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461–2. DOIPubMedGoogle Scholar
- Mylotte JM. Antimicrobial prescribing in long-term care facilities: prospective evaluation of potential antimicrobial use and cost indicators. Am J Infect Control. 1999;27:10–9. DOIPubMedGoogle Scholar
- McDonald LC, Owings M, Jernigan D. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12:409–15. DOIPubMedGoogle Scholar
- Galdys AL, Nelson JS, Shutt KA, Schlackman JL, Pakstis DL, Pasculle AW, Prevalence and duration of asymptomatic Clostridium difficile carriage among healthy subjects in Pittsburgh, Pennsylvania. J Clin Microbiol. 2014;52:2406–9. DOIPubMedGoogle Scholar
- Alasmari F, Seiler SM, Hink T, Burnham C-AD, Dubberke ER. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis. 2014;59:216–22. DOIPubMedGoogle Scholar
- Loo VG, Bourgault A-M, Poirier L, Lamothe F, Michaud S, Turgeon N, Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–703. DOIPubMedGoogle Scholar
- Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. Equation of state calculations by fast computing machines. J Chem Phys. 1953;21:1087. DOIGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 22, Number 4—April 2016
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
David P. Durham, Center for Infectious Disease Modeling and Analysis, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, 135 College St, Ste 200, New Haven, CT 06520, USA
Top