Volume 22, Number 6—June 2016
Synopsis
Improved Global Capacity for Influenza Surveillance
Figure 2
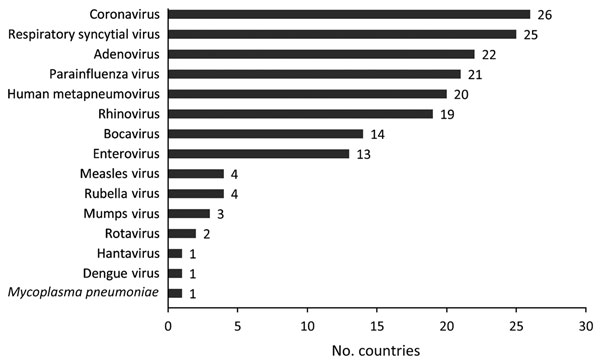
Figure 2. Number of countries that reported adding different virology testing assays to routine influenza laboratory testing platform by virus type from the start of the partnership program with the Centers for Disease Control and Prevention to strengthen influenza surveillance capacity, 2004–2013. From a total of 39 participating countries, 35 responded to a 2013 questionnaire; 28 reported adding tests for other pathogens.
Page created: August 18, 2016
Page updated: August 18, 2016
Page reviewed: August 18, 2016
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.