Volume 23, Number 3—March 2017
Dispatch
Likely Autochthonous Transmission of Trypanosoma cruzi to Humans, South Central Texas, USA
Abstract
Chagas disease, caused by Trypanosoma cruzi, is a major neglected tropical disease affecting the Americas. The epidemiology of this disease in the United States is incomplete. We report evidence of likely autochthonous vectorborne transmission of T. cruzi and health outcomes in T. cruzi–seropositive blood donors in south central Texas, USA.
Chagas disease (Trypanosoma cruzi infection) is a neglected tropical disease affecting the Americas and a major cause of preventable illness and death, with ≈6–8 million cases worldwide (1). This disease can cause progressive cardiac damage postinfection in 30% of infected persons without any initial suggestive clinical symptoms. These latent infections can remain quiescent for decades before manifesting as cardiac complications, including cardiomyopathy, heart failure, and rare cardiac arrest (2).
In 2010, US Food and Drug Administration (Sil-ver Springs, MD, USA) issued final guidelines regard-ing screening of the US blood supply for T. cruzi (3,4). During 2008–2012, screening results showed that 1 in 6,500 donors from an area covering most of the state of Texas were reactive for T. cruzi antibodies (5). The origin of infection for these donors was unknown. How-ever, high infection rates for reservoir animals and triato-mine bug vectors in south central Texas suggested that T. cruzi transmission cycles resulting in human infections could occur at a higher frequency than suspected (6,7). Therefore, we evaluated potential transmission sources and cardiac health of blood donors from south central Texas with T. cruzi antibodies.
This study was approved by institutional review boards at Baylor College of Medicine (Houston, TX, USA), the Gulf Coast Regional Blood Center (Houston), and the South Texas Tissue and Blood Center (San Antonio, TX, USA). Blood donors residing in the greater San Antonio, Texas, area who had T. cruzi antibodies detected by PRISM Chemiluminescent Immunoassay (Abbott Laboratories, Chicago, IL, USA) or Ortho T. cruzi ELISA (Ortho-Clinical Diagnostics Inc., Raritan, NJ, USA), and a positive result for a Radioimmune Precipitation Assay (Quest Diagnostic Laboratories, Madison, NJ, USA) or an ESA Chagas Test (Abbott Laboratories) during January 1, 2008–December 31, 2014, were invited to participate in the study. Persons previously enrolled in a Houston-based T. cruzi–seropositive blood donor project were not eligible for this study (8).
Letters in English and Spanish were sent to donors who had T. cruzi antibodies by the blood centers for this study. Those who agreed to participate provided informed consent. We performed 3 procedures: 1) blood collection for additional serologic screening, 2) structured interview to assess potential transmission sources and health, and 3) 12-lead resting electrocardiogram (ECG) (8).
Blood specimens were used for serologic testing (Table 1). We defined a case of T. cruzi infection if donor screening test results and >2 serologic test results were positive. Likely autochthonous T. cruzi infection was defined in a case-patient who had no major travel to a Latin American country (lasting >2 weeks or that included an overnight stay in a rural region), not having been born in Latin America, and not having a mother born in Latin America (4,8–10). Congenital transmission from a maternal grandmother (2 contiguous congenital infections) cannot be ruled out with this case definition but is unlikely given the low risk for congenital transmission (7). Occupations, residential history, and clinical health information were reviewed in a questionnaire. ECG readings were interpreted by a board-certified cardiologist.
For persons who donated blood in the greater San Antonio area during the study period, we found that 61/256,801 donors had positive serologic results for T. cruzi infection (1/4,200 donors had positive serologic results for T. cruzi infection by 2 assays). Seventeen (28%) of these were enrolled in the study; additional serologic testing confirmed that 14 had antibodies against T. cruzi when the study began (Table 1). These persons had a mean age of 47 years (range 19–83 years); 50% were Hispanic, 50% were non-Hispanic white, and 50% were men. For 3 persons whose blood donor testing results were not confirmed by further serologic testing, 2 were non-Hispanic and 1 was Hispanic (2 women and 1 man); mean age was 51 years. Because of the blinded nature of study recruitment, we cannot identify demographic data for persons who received the letter and chose not to participate.
Likely autochthonous transmission of T. cruzi was suspected for 11 (79%) of 14 persons, as defined by study criteria. These 11 persons had a mean age of 50 years; 7 were non-Hispanic whites, and 6 were men. Remaining data presented will concern only the 11 newly identified persons with likely autochthonous infections.
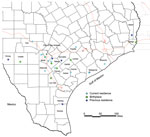
A structured interview adapted from a questionnaire used by the Centers for Disease Control and Prevention (Atlanta, GA, USA), the American Red Cross (Washington, DC, USA), and Blood Systems, Inc. (Scottsdale, AZ, USA), was used to identify risk factors for T. cruzi infection (4). Because of the lifelong nature of infection and antibody-based diagnostics used, a specific time of infection could not be established for each case-patient. However, we identified common themes for transmission risks for this cohort. Most (91%) case-patients with likely autochthonous infection reported a history of living in a rural community (Figure). Residence in rural communities could pose a risk for T. cruzi transmission because this setting might lead to close proximity with sylvatic transmission cycles involving the vector and infected animals (11).
Although recreational activities or occupations associated with outdoor exposure were reported among our cohort, we obtained evidence suggesting that opportunities for transmission might be occurring near homes in rural communities (Table 2). Specifically, patients with likely autochthonous infections reported seeing the vector around their current or previous residence (36%), and had animal housing near their homes (73%). An extensive history of outdoor recreational activities of hunting and camping, which has been suggested as a high-risk activity for T. cruzi transmission in the southern United States, was less common then expected (36%) (8,12). Two of 11 case-patients reported agricultural jobs and staying in substandard housing during the harvest season, thereby introducing the potential for disease transmission from triatomines in the home.
Five case-patients reported a lack of knowledge of Chagas disease by their primary care physicians. Some case-patients were provided with misinformation, reporting having been told that their screening test result must be false positive because they had no travel history. Furthermore, only 2 case-patients were offered treatment before enrollment in the study. One case-patient reported that, despite seeking treatment for >1 year, he was unable to find a physician able and willing to help.
This finding is particularly problematic given that a large proportion (6 of 11) of this cohort had abnormal ECG readings possibly attributable to Chagasic cardiac disease. Although precise cardiac etiologies could not be determined, prevalence of ECG abnormalities was higher than that for population-based studies (13,14); common findings included atrioventricular block and left axis deviation (Table 1). A previous report also highlighted the same lack of physician awareness of Chagas disease in Texas, despite patients having positive serologic screening results and cardiac manifestations (8).
Given the low level of participation of seropositive blood donors, results of this study are limited to persons who participated and might not represent the larger Texas blood donor population or general population. Also, because a 7-year span separated initial screening and enrollment in this study, it is difficult to identify why 3 persons who were initially positive by blood bank screening had discordant results during the study. At follow-up, participating persons were tested with available Centers for Disease Control and Prevention assays, Food and Drug Administration–approved screening, or supplemental tests.
Our study adds 11 cases of likely domestically acquired T. cruzi infection to the increasing body of evidence for autochthonous Chagas disease transmission in the southern United States. Combined with previous studies indicating a high rate of T. cruzi infection in triatomine vectors and mammalian reservoirs in this area, our study shows that south central Texas could be a focal point for endemic disease transmission (7,15). We also identified a major knowledge gap for Chagas disease, which highlights the need for enhanced public health campaigns targeting clinicians and the general population in south central Texas.
Dr. Gunter is a postdoctoral fellow at Baylor College of Medicine, Houston, TX. Her research interests include the epidemiology of Chagas disease in the United States and host–parasite interaction.
Acknowledgment
This study was supported by a private donation to the Texas Children’s Hospital and Baylor College of Medicine.
References
- Chagas disease. 2014 [cited 2016 Apr 6]. http://www.paho.org/hq/index.php?option=com_content&view=article&id=5856&Itemid=41506&lang=en
- Sabino EC, Ribeiro AL, Salemi VM, Di Lorenzo Oliveira C, Antunes AP, Menezes MM, et al.; National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II), International Component. Ten-year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi-seropositive former blood donors. Circulation. 2013;127:1105–15. DOIPubMedGoogle Scholar
- US Department of Health and Human Services Food and Drug Administration. Guidance for industry: use of serological tests to reduce the risk of transmission of Trypanosoma cruzi infection in whole blood and blood components intended for transfusion. Washington (DC) and Silver Spring (MD): The Department and the Administration; 2010.
- Cantey PT, Stramer SL, Townsend RL, Kamel H, Ofafa K, Todd CW, et al. The United States Trypanosoma cruzi Infection Study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion. 2012;52:1922–30. DOIPubMedGoogle Scholar
- Garcia MN, Woc-Colburn L, Rossmann SN, Townsend RL, Stramer SL, Bravo M, et al. Trypanosoma cruzi screening in Texas blood donors, 2008-2012. Epidemiol Infect. 2016;144:1010–3. DOIPubMedGoogle Scholar
- Curtis-Robles R, Wozniak EJ, Auckland LD, Hamer GL, Hamer SA. Combining public health education and disease ecology research: using citizen science to assess Chagas Disease entomological risk in Texas. PLoS Negl Trop Dis. 2015;9:e0004235. DOIPubMedGoogle Scholar
- Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011;24:655–81. DOIPubMedGoogle Scholar
- Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, et al. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg. 2015;92:325–30. DOIPubMedGoogle Scholar
- Leiby DA, Read EJ, Lenes BA, Yund AJ, Stumpf RJ, Kirchhoff LV, et al. Seroepidemiology of Trypanosoma cruzi, etiologic agent of Chagas’ disease, in US blood donors. J Infect Dis. 1997;176:1047–52. DOIPubMedGoogle Scholar
- Wilson LS, Ramsey JM, Koplowicz YB, Valiente-Banuet L, Motter C, Bertozzi SM, et al. Cost-effectiveness of implementation methods for ELISA serology testing of Trypanosoma cruzi in California blood banks. Am J Trop Med Hyg. 2008;79:53–68.PubMedGoogle Scholar
- Sarkar S, Strutz SE, Frank DM, Rivaldi CL, Sissel B, Sánchez-Cordero V. Chagas disease risk in Texas. PLoS Negl Trop Dis. 2010;4:e836. DOIPubMedGoogle Scholar
- Garcia MN, Hotez PJ, Murray KO. Potential novel risk factors for autochthonous and sylvatic transmission of human Chagas disease in the United States. Parasit Vectors. 2014;7:311. DOIPubMedGoogle Scholar
- De Bacquer D, De Backer G, Kornitzer M. Prevalences of ECG findings in large population based samples of men and women. Heart. 2000;84:625–33. DOIPubMedGoogle Scholar
- Ribeiro AL, Sabino EC, Marcolino MS, Salemi VM, Ianni BM, Fernandes F, et al.; NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II), International Component. Electrocardiographic abnormalities in Trypanosoma cruzi seropositive and seronegative former blood donors. PLoS Negl Trop Dis. 2013;7:e2078. DOIPubMedGoogle Scholar
- Kjos SA, Marcet PL, Yabsley MJ, Kitron U, Snowden KF, Logan KS, et al. Identification of bloodmeal sources and Trypanosoma cruzi infection in triatomine bugs (Hemiptera: Reduviidae) from residential settings in Texas, the United States. J Med Entomol. 2013;50:1126–39. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 23, Number 3—March 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Melissa N. Garcia, Baylor College of Medicine, 1102 Bates Ave, Ste 550.02, Houston, TX 77030, USA
Top