Volume 24, Number 4—April 2018
Dispatch
mcr-1 in Carbapenemase-Producing Klebsiella pneumoniae with Hospitalized Patients, Portugal, 2016–2017
Figure 1
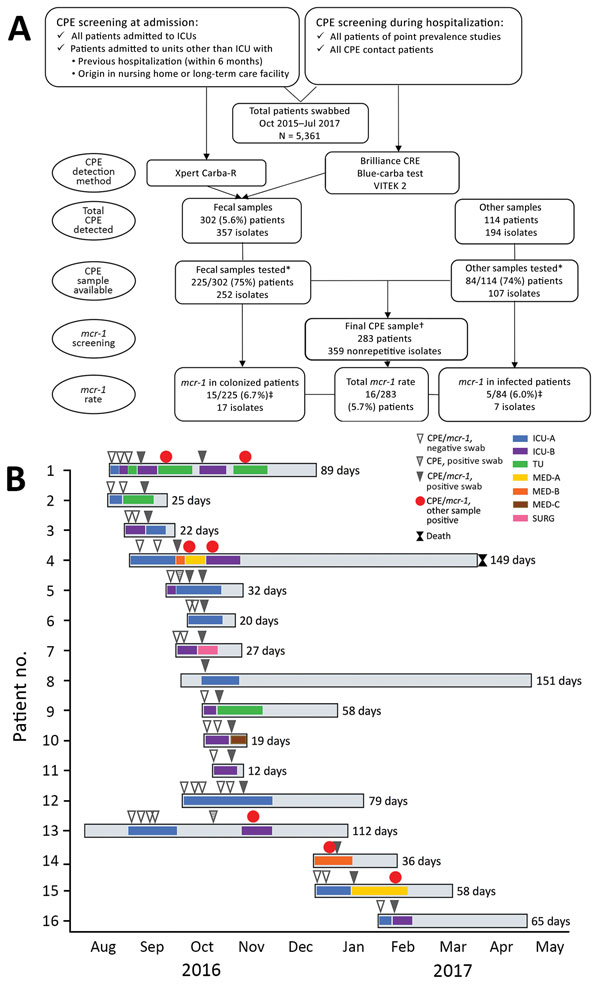
Figure 1. Selection for and testing of patients with Klebsiella pneumoniae carbapenemase 3–producing mcr-1–positive Enterobacteriaceae, Porto, Portugal, 2016–2017. A) Flowchart demonstrating rationale for sample selection. First, we screened for asymptomatic carriage of CPE in the gastrointestinal tract (i.e., colonization by CPE) by testing patient fecal samples with Brilliance CRE Agar (Oxoid, Basingstoke, UK); Xpert Carba-R (Cepheid, Sunnyvale, CA, USA); and VITEK 2 (bioMérieux, Marcy l’Etoile, France). Second, we tested for CPE with all patient samples available. Last, we screened the carbapenemase-producing isolates for mcr-1 to identify the final sample. *CPE isolates and complete epidemiologic and clinical data were available for ≈75% of CPE patients. †The final sample screened for mcr-1 included only nonrepetitive isolates. For fecal samples, we considered isolates repetitive when detected in the same patient in samples collected within 72 h from each other. For other types of samples, we considered isolates repetitive when detected in the same sample type collected at the same time point. ‡Four patients carried mcr-1–positive isolates either in the gastrointestinal tract or in other body sites. B) Timeline representing epidemiologic data of the 16 patients with mcr-1–positive CPE. CPE, carbapenemase-producing Enterobacteriaceae; ICU, intensive care unit; MED, medical unit; SURG, surgical unit; TU, transplant unit.
1These authors contributed equally to this article.