Volume 24, Number 5—May 2018
Dispatch
External Quality Assessment for Zika Virus Molecular Diagnostic Testing, Brazil
Figure 1
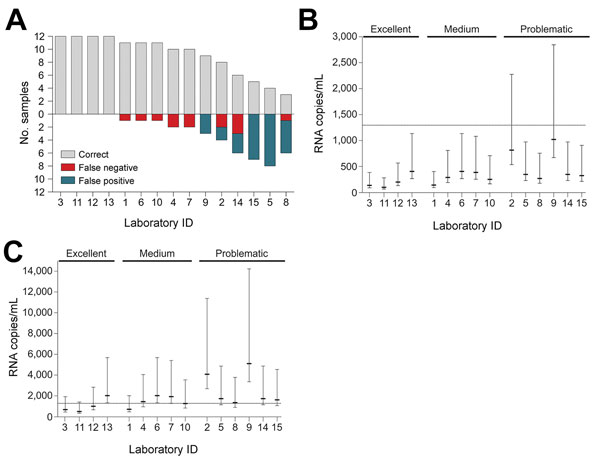
Figure 1. External quality assessment (EQA) performance and lower limits of detection (LODs) for Zika virus molecular diagnostic testing, Brazil. A) EQA performance of individual laboratories. Gray bars above the baseline indicate correctly tested samples; bars below the baseline indicate incorrectly tested samples. Laboratories are sorted by the quantity of correct samples and the numeric order of the laboratory identification numbers. Laboratory 8 tested only 9 of 12 samples. B) Projected 95% LODs of participating laboratories under optimal conditions; C) projected 95% LODs of participating laboratories assuming a 5-fold loss in sensitivity. LODs were projected using the technical LOD of the Lanciotti et al. assay as analyzed previously (2), input and elution volumes, and real-time reverse transcription-PCR setups. Efficacy of RNA extraction was assumed to be 100%. Whiskers indicate 95% CIs. Dotted line indicates the lowest Zika virus RNA titer of an EQA specimen. Laboratories are grouped according to their EQA performance as excellent, medium, or problematic. LODs did not differ significantly among groups (p>0.05 by Kruskal-Wallis test)..
References
- de Oliveira WK, de França GVA, Carmo EH, Duncan BB, de Souza Kuchenbecker R, Schmidt MI. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet. 2017;390:861–70. DOIPubMedGoogle Scholar
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–9. DOIPubMedGoogle Scholar
- Corman VM, Rasche A, Baronti C, Aldabbagh S, Cadar D, Reusken CB, et al. Assay optimization for molecular detection of Zika virus. Bull World Health Organ. 2016;94:880–92. DOIPubMedGoogle Scholar
- Brasil P, Pereira JP Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321–34. DOIPubMedGoogle Scholar
- de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo APL, et al.; investigators from the Microcephaly Epidemic Research Group; Brazilian Ministry of Health; Pan American Health Organization; Instituto de Medicina Integral Professor Fernando Figueira; State Health Department of Pernambuco. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16:1356–63. DOIPubMedGoogle Scholar
- Charrel R, Mögling R, Pas S, Papa A, Baronti C, Koopmans M, et al. Variable sensitivity in molecular detection of Zika virus in European expert laboratories: external quality assessment, November 2016. J Clin Microbiol. 2017;55:3219–26. DOIPubMedGoogle Scholar
- Baylis SA, Hanschmann KO, Schnierle BS, Trösemeier JH, Blümel J; Zika Virus Collaborative Study Group. Harmonization of nucleic acid testing for Zika virus: development of the 1stWorld Health Organization International Standard. Transfusion. 2017;57(3pt2):748–61. DOIPubMedGoogle Scholar
- Halai UA, Nielsen-Saines K, Moreira ML, de Sequeira PC, Junior JPP, de Araujo Zin A, et al. Maternal Zika virus disease severity, virus load, prior dengue antibodies, and their relationship to birth outcomes. Clin Infect Dis. 2017;65:877–83. DOIPubMedGoogle Scholar
- Terzian ACB, Schanoski AS, Mota MTO, da Silva RA, Estofolete CF, Colombo TE, et al. Viral load and cytokine response profile does not support antibody-dependent enhancement in dengue-primed Zika virus–infected patients. Clin Infect Dis. 2017;65:1260–5. DOIPubMedGoogle Scholar
- Domingo C, Escadafal C, Rumer L, Méndez JA, García P, Sall AA, et al. First international external quality assessment study on molecular and serological methods for yellow fever diagnosis. PLoS One. 2012;7:e36291. DOIPubMedGoogle Scholar
- Domingo C, Niedrig M, Teichmann A, Kaiser M, Rumer L, Jarman RG, et al. 2nd International external quality control assessment for the molecular diagnosis of dengue infections. PLoS Negl Trop Dis. 2010;4:e833. DOIPubMedGoogle Scholar
- Niedrig M, Linke S, Zeller H, Drosten C. First international proficiency study on West Nile virus molecular detection. Clin Chem. 2006;52:1851–4. DOIPubMedGoogle Scholar
- Drexler JF, Kupfer B, Petersen N, Grotto RM, Rodrigues SM, Grywna K, et al. A novel diagnostic target in the hepatitis C virus genome. PLoS Med. 2009;6:e31. DOIPubMedGoogle Scholar
- Moreira-Soto A, Sarno M, Pedroso C, Netto EM, Rockstroh A, Luz E, et al. Evidence for congenital Zika virus infection from neutralizing antibody titers in maternal sera, north-eastern Brazil. J Infect Dis. 2017;216:1501–4. DOIPubMedGoogle Scholar
- Aiken AR, Scott JG, Gomperts R, Trussell J, Worrell M, Aiken CE. Requests for abortion in Latin America related to concern about Zika virus exposure. N Engl J Med. 2016;375:396–8. DOIPubMedGoogle Scholar