Volume 24, Number 5—May 2018
Research Letter
Chronic Genotype 3 Hepatitis E in Pregnant Woman Receiving Infliximab and Azathioprine
Figure
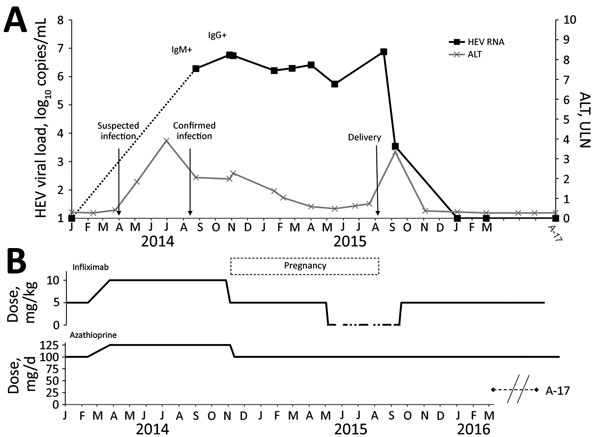
Figure. Time courses of HEV viral load (A) and infliximab and azathioprine treatment (B) in pregnant woman with chronic hepatitis E who was undergoing immunosuppressive treatment for ulcerative colitis. HEV RNA levels, IgM and IgG positivity, and serum ALT levels are shown. Serum ALT is expressed as a multiple of ULN. Arrows indicate suspected and confirmed infection and delivery dates. Infliximab treatment occurred every 8 weeks. ALT, alanine aminotransferase; HEV, hepatitis E virus; ULN, upper limit of normal.
Page created: April 17, 2018
Page updated: April 17, 2018
Page reviewed: April 17, 2018
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.