Outcomes of Bedaquiline Treatment in Patients with Multidrug-Resistant Tuberculosis
Lawrence Mbuagbaw
, Lorenzo Guglielmetti, Catherine Hewison, Nyasha Bakare, Mathieu Bastard, Eric Caumes, Mathilde Fréchet-Jachym, Jérôme Robert, Nicolas Veziris, Naira Khachatryan, Tinatin Kotrikadze, Armen Hayrapetyan, Zaza Avaliani, Holger J. Schünemann, and Christian Lienhardt
Author affiliations: St. Joseph’s Healthcare Hamilton, Hamilton, Ontario, Canada (L. Mbuagbaw); Centre for the Development of Best Practices in Health, Yaoundé, Cameroon (L. Mbuagbaw); McMaster University, Hamilton (L. Mbuagbaw, H.J. Schünemann); Centre d'Immunologie et des Maladies Infectieuses, INSERM, Paris (L. Guglielmetti, E. Caumes, J. Robert, N. Veziris); Centre Hospitalier de Bligny, Bris-sous-Forges, France (L. Guglielmetti, M. Fréchet-Jachym); Sorbonne Université, Paris, France (L. Guglielmetti, J. Robert, N. Veziris); Médecins Sans Frontières, Paris (C. Hewison); Janssen Research & Development, LLC, Titusville, New Jersey, USA (N. Bakare); Epicentre, Paris (M. Bastard); Hôpitaux Universitaires de l'Est Parisien, Paris (N. Veziris); Médecins Sans Frontières, Yerevan, Armenia (N. Khachatryan); Médecins Sans Frontières, Tbilisi, Georgia (T. Kotrikadze); National Tuberculosis Control Centre, Yerevan (A. Hayrapetyan); National Centre for Tuberculosis and Lung Disease, Tbilisi (Z. Avaliani); World Health Organization, Geneva, Switzerland (C. Lienhardt); Université de Montpellier, Montpellier, France (C. Lienhardt)
Main Article
Figure
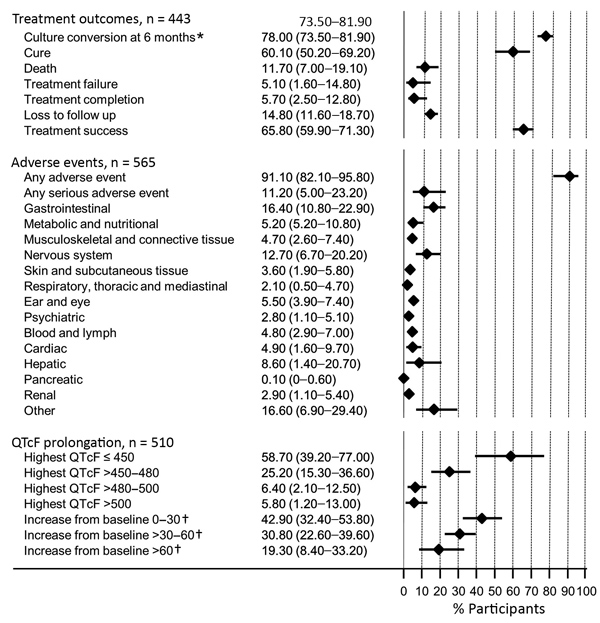
Figure. Summary of treatment outcomes and adverse events in study of bedaquiline treatment for multidrug-resistant tuberculosis. Values are shown as percent with 95% CI, shown in the graph as horizontal bars. QTcF indicates QT intervals corrected for heart rate using the Fridericia formula. * A total of 406 study participants with culture data at the 6-month point; †, a total of 509 participants with baseline QTcF data.
Main Article
Page created: April 18, 2019
Page updated: April 18, 2019
Page reviewed: April 18, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
