Volume 25, Number 8—August 2019
Research Letter
Sneathia amnii and Maternal Chorioamnionitis and Stillbirth, Mozambique
Abstract
We report a case of Sneathia amnii as the causative agent of maternal chorioamnionitis and congenital pneumonia resulting in a late fetal death in Mozambique, with strong supportive postmortem molecular and histopathologic confirmation. This rare, fastidious gram-negative coccobacillus has been reported to infrequently cause abortions, stillbirths, and neonatal infections.
Sneathia amnii, formerly designated Leptotrichia amnionii, is a rare, fastidious, gram-negative coccobacillus, first described in the amniotic fluid of a woman with a fetal demise (1). The inherent difficulties in conventionally culturing this pathogen led to its initial identification through analyzing the 16S rRNA gene; its genome was recently sequenced (1,2). S. amnii is an opportunistic agent of the female urogenital tract (3,4) associated with cases of spontaneous abortion (miscarriage) and neonatal meningitis (1,5,6). We describe a perinatal case of S. amnii infection in a mother–fetus dyad, which we documented and investigated with the minimally invasive tissue sampling (MITS) postmortem procedure (7).
An otherwise healthy multigravida 37-year-old woman, at an estimated gestational age of 39 weeks, was admitted to Manhiça District Hospital, southern Mozambique, in labor. During pregnancy, she had attended 2 antenatal consultations and received the standard of care for pregnant women in Mozambique; mild anemia was treated with ferrous sulfate and folic acid supplements. Serologic tests for syphilis and HIV were both negative. Upon arrival at the hospital, the mother was afebrile and hemodynamically stable; she had a fully effaced uterine cervix, thin and elastic, 2 cm dilation; intact amniotic membranes; and cephalic fetal presentation with heartbeat present. Physical examination did not provide additional information. Labor progressed with spontaneous rupture of membranes. No additional documentation of the fetal heartbeat was available before delivery. Two hours after arrival, a fresh stillborn female weighing 3.5 kg was born by spontaneous vaginal delivery. Size was normal, and no macroscopic congenital abnormalities were observed. The mother was discharged next day without complications.
As part of Mozambique’s Child Health and Mortality Prevention Surveillance (CHAMPS), after obtaining written, informed consent, we conducted MITS by biopsy needle of tissues and body fluids, in addition to placenta, to ascertain the cause of the stillbirth (7). Samples are subject to thorough histopathologic, molecular, and microbiological investigation, including universal screening for HIV-1, Mycobacterium tuberculosis, and malaria parasites. We performed conventional microbiological cultures of blood and cerebrospinal fluid (CSF); we inoculated ≈3 mL of blood into aerobic blood culture bottle (BACTEC system; Becton Dickinson, https://www.bd.com) and cultured CSF samples into blood, chocolate, and MacConkey agar plates. We performed multipathogen molecular screening using TaqMan Array Card (Applied Biosystems, https://www.thermofisher.com) in whole blood, CSF, lung, and rectal swab samples (8). We prepared and examined tissue samples using conventional pathologic methods and targeted immunohistochemical staining (9).
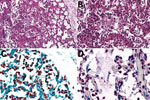
We isolated no microorganisms in CSF or blood, nor did we detect a likely pathogen in any of the unfixed postmortem tissues. At CHAMPS reference pathology laboratories, examination of tissue samples showed similar morphological findings in placental and miscellaneous tissues that suggested infection, including an acute inflammatory infiltrate in the lungs compatible with bronchopneumonia. We also found moderate numbers of aspirated squames and increased alveolar macrophages, indicating intrauterine fetal distress and associated aspiration of amniotic fluid. No aspirated meconium was apparent. Gram stain revealed gram-negative coccobacilli in alveoli and adjacent bronchioles. We conducted a cross-reactive immunohistochemical assay targeting multiple bacteria in the lung samples using paneubacteria and gram type–specific PCR assays targeting the 16S rRNA gene; we identified S. amnii by sequence analysis of positive amplicons (Figure, panels A–C). We observed no remarkable histopathologic findings in the liver or brain, and the cross-reactive polybacterial immunohistochemical assay was negative in brain tissue. Placental tissue and umbilical cord showed an acute chorioamnionitis with maternal response (inflammation in the membranes, stage 2) and fetal response (inflammation in the umbilical cord, stage 2) showing umbilical arteritis with rare gram-negative coccobacilli. There was no immunohistochemical evidence of bacteria in this tissue (Figure, panel D). We obtained an amplicon from placental tissue by paneubacteria PCR; however, we could not confirm the presence of S. amnii sequences.
CHAMPS procedures include the review of all clinical, microbiological, molecular, and histopathological data, along with the verbal autopsy, by a multidisciplinary panel of local experts (D.M. Blau et al., unpub. data). The panel concluded that the immediate cause of this stillbirth could be attributed to a congenital pneumonia, caused by S. amnii, that could have originated in the mother’s placenta; we determined that chorioamnionitis was the main maternal condition associated with the child’s death. The presence of Sneathia sp. bacteria in amniotic fluid can lead to inflammation and histologic chorioamnionitis, amnionitis, or both (10).
S. amnii has been identified in different settings as a pathologic agent in women and children (1,3–6). In this case in a rural setting in Africa, S. amnii was the causative agent in a stillbirth with congenital pneumonia, a diagnosis supported by strong postmortem molecular and histopathologic confirmation. As CHAMPS evaluation continues in Mozambique, as well as at sites in 6 additional countries in sub-Saharan Africa and south Asia, we expect the importance of this pathogen to become clearer.
Dr. Vitorino is a clinical researcher at Centro de Investigação em Saúde de Manhiça, Maputo, Mozambique. Her research interests are in pediatric infectious diseases and key determinants of pediatric causes of death in resource-constrained settings. Dr. Varo is a medical research fellow with ISGlobal whose interests include malaria clinical trials and key determinants of pediatric causes of death in resource-constrained settings.
Acknowledgments
The Spanish Agency of Cooperation and International Development (AECID) funds the core activities of Centro de Investigação em Saúde de Manhiça. R.V. had a fellowship from the Rio Hortega program of the Instituto de Salud Carlos III (ISCIII) grant no. CD16/00024.
J.M.G. received grants from the Gates Foundation during the study period. The other authors declare that they have no competing interests.
References
- Shukla SK, Meier PR, Mitchell PD, Frank DN, Reed KD. Leptotrichia amnionii sp. nov., a novel bacterium isolated from the amniotic fluid of a woman after intrauterine fetal demise. J Clin Microbiol. 2002;40:3346–9. DOIPubMedGoogle Scholar
- Harwich MD Jr, Serrano MG, Fettweis JM, Alves JM, Reimers MA, Buck GA, et al.; Vaginal Microbiome Consortium (additional members). Genomic sequence analysis and characterization of Sneathia amnii sp. nov. BMC Genomics. 2012;13(Suppl 8):S4. DOIPubMedGoogle Scholar
- Gundi VA, Desbriere R, La Scola B. Leptotrichia amnionii and the female reproductive tract. Emerg Infect Dis. 2004;10:2056–7. DOIPubMedGoogle Scholar
- Thilesen CM, Nicolaidis M, Lökebö JE, Falsen E, Jorde AT, Müller F. Leptotrichia amnionii, an emerging pathogen of the female urogenital tract. J Clin Microbiol. 2007;45:2344–7. DOIPubMedGoogle Scholar
- Boennelycke M, Christensen JJ, Arpi M, Krause S. Leptotrichia amnionii found in septic abortion in Denmark. Scand J Infect Dis. 2007;39:382–3. DOIPubMedGoogle Scholar
- Decroix V, Goudjil S, Kongolo G, Mammeri H. ‘Leptotrichia amnionii’, a newly reported cause of early onset neonatal meningitis. J Med Microbiol. 2013;62:785–8. DOIPubMedGoogle Scholar
- Menendez C, Castillo P, Martínez MJ, Jordao D, Lovane L, Ismail MR, et al. Validity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: An observational study. PLoS Med. 2017;14:
e1002318 . DOIPubMedGoogle Scholar - Diaz MH, Waller JL, Theodore MJ, Patel N, Wolff BJ, Benitez AJ, et al. Development and implementation of multiplex TaqMan array cards for specimen testing at Child Health and Mortality Prevention Surveillance (CHAMPS) site laboratories. Clin Infect Dis. 2019. In press.
- Martines RB, Ritter JM, Gary J, Shieh W-J, Ordi J, Hale M, et al. Pathology and telepathology methods in the Child Health and Mortality Prevention Surveillance Network (CHAMPS). Clin Infect Dis. 2019. In press.
- Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: July 06, 2019
1These authors contributed equally to this article.
Table of Contents – Volume 25, Number 8—August 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Rosauro Varo, Centro de Investigação em Saúde de Manhiça, Clinical Department, Rua 12 Cambeve, Manhica, Maputo 1919, Mozambique
Top