Volume 26, Number 10—October 2020
Dispatch
Seawater-Associated Highly Pathogenic Francisella hispaniensis Infections Causing Multiple Organ Failure
Abstract
A rare case of Francisella hispaniensis infection associated with seawater exposure occurred in a deep-sea diving fisherman in Zhejiang, China. He had skin and soft tissue infection that progressed to bacteremia and multiple organ failure. Moxifloxacin treatment cleared the infections, but the patient suffered a sequela of heart damage.
Francisella tularensis, the agent of tularemia, is an important human pathogen (1). Other Francisella species, such as F. philomiragia, mainly associated with saltwater exposure, rarely also cause human infections (2). F. hispaniensis, first isolated from the blood of a patient in Spain (3), is an emerging human pathogen, but its epidemiology and pathogenicity remain a mystery because only 2 cases have been reported (3,4). We report a case of F. hispaniensis infection in China.
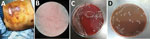
On September 6, 2018, a 64-year-old male fisherman sought care for a prominent cutaneous ulcer on the right lower chest, chest pain, and fever for 16 days and was admitted to The First Affiliated Hospital of Zhejiang University (Hangzhou, China). He was previously healthy without remarkable medical history. He worked as a deep-sea diving fisherman in Sanmen Bay, Taizhou, a coastal city in Zhejiang Province, adjoining the East China Sea. A superficial wound progressed to cellulitis in the right lower chest after a deep-sea dive without protective clothing. Low-grade fever and chest pain then developed. He received amoxicillin/clavulanic acid at a local clinic, and his fever resolved after 2 days. Because he felt better, he stopped taking the amoxicillin/clavulanic acid and resumed deep-sea diving. Two days later, his chest wound had worsened with purulent discharge, and his low-grade fever returned. Twelve days later he sought care at another hospital because of high fever and respiratory distress. He received 2 days of ceftizoxime followed by imipenem for 7 days, but his condition deteriorated, and irritability, chest tightness, nausea, vomiting, abdominal distension, chills, and high fever (39.4°C) developed. At admission to The First Affiliated Hospital of Zhejiang University School of Medicine, he had sepsis, hypotension, and leukocytosis and immediately received norepinephrine intravenous pumping, endotracheal intubation, sedation, mechanical ventilation, and continuous renal replacement therapy. His lower chest showed a large ulcer with bleeding, purulent discharge, and tissue necrosis (Figure 1, panel A). Laboratory test results showed highly elevated inflammatory markers, acidosis, coagulopathy, and elevated liver enzymes, bilirubin, creatinine, and troponin (Table 1). Chest computed tomography scan showed right lower lobe consolidation, pleural effusion in the right thoracic cavity, and multiple calcified lymph nodes in the mediastinum. Abdomen computed tomography scan showed hepatosplenomegaly and effusion in the abdominal and pelvic cavities. Echocardiography showed decreased left ventricular systolic function and diffuse abnormal movement of left ventricular wall. Electrocardiograph showed cardiac arrhythmia with sinus bradycardia, ventricular premature beats, and paroxysmal ventricular tachycardia. Acute diffuse myocarditis was diagnosed and prompted dobutamine treatment.
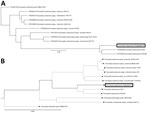
Blood, pleural fluid, and wound culture all grew gram-negative cocci (Figure 1, panel B), identified by Vitek2 (bioMérieux, https://www.biomerieux.com) as Sphingomonas paucimobilis. The bacteria grew well on the regular sheep blood agar and showed medium-sized, smooth-edged, mucoid and greyish white colonies (Figure 1, panel C). They grew better on chocolate agar (Figure 1, panel D) but did not grow on MacConkey agar. The bacteria were catalase weakly positive, oxidase positive, indole negative, and β-lactamase positive. Because S. paucimobilis is usually considered an environmental bacterium and unlikely to cause such severe systemic infections, we sent the patient’s blood for shotgun metagenomic sequencing test and the bacterial isolate for whole-genome sequencing (WGS) using Illumina MiniSeq (https://www.illumina.com). Metagenomic sequencing yielded a positive result as F. tularensis, but WGS identified F. hispaniensis, on the basis of k-mer and single-nucleotide polymorphism phylogenetic tree analyses performed using CLCbio (QIAGEN, https://www.qiagen.com) (Figure 2), which showed the bacteria clustered closely with 2 other F. hispaniensis strains (3,4) and very distantly with other Francisella species. To verify the results, we mapped the raw sequencing reads to the most closely related reference genome F. hispaniensis FSC454 (GenBank accession no. CP018093) using Geneious (BioMatters, https://www.geneious.com), which resulted in 96.1% genome coverage with 97.9% pairwise identity. The FSC454 and Zhejiang2018 strains differ by only 1 nt (A1029G) in the 16S rRNA gene (99.94% identity) and 10 nt changes in the recA gene (99.07% identity).
Drug susceptibility tests showed resistance to colistin, trimethoprim/sulfamethoxazole, third-generation cephalosporins, and carbapenems but susceptibility to piperacillin/tazobactam, cefepime, fluoroquinolones, aminoglycosides, and tetracyclines (Table 2). Because a Bla-2/FTU-1 class-A β-lactamase is expressed among most Francisella species (6), the strain reported here also carries a homologue gene of 867 bp with 89.7% identity to the reference gene (GenBank accession no. NG_049110_FTU-1) (7). No plasmids were identified. Other resistance genes identified were aph(3¢)-Ia, predicting resistance to kanamycin; mdf(A), predicting resistance to macrolide; and catA1, predicting resistance to phenicol. However, broth microdilution tests showed low MIC for kanamycin, erythromycin, azithromycin, and chloramphenicol (Table 2). The reason for the inconsistency between the resistance genes detected and phenotypic susceptibility results is unclear and requires further investigation.
On the basis of the MIC results and the literature (4,8,9), we chose moxifloxacin (400 mg 1×/d injection) to treat the infection. After 14 days of treatment, the patient’s symptoms markedly improved, and the chest wound started to heal. Most blood test results had returned to normal ranges (Table 1). However, his heart suffered long-term damage because of the myocarditis, and he required a pacemaker. He was discharged 28 days after admission.
In the 2 previously reported human F. hispaniensis infections, the bacteria were isolated from blood (3,4). The patient we report first suffered a trauma to unprotected skin of his chest that was exposed to seawater from which the bacteria entered the wound and caused the skin and soft tissue infections that progressed to bacteremia and sepsis. Like F. tularensis, F. hispaniensis appeared to be highly pathogenic and caused respiratory failure, septic shock, and multiple organ dysfunction syndrome. Unlike F. tularensis, F. hispaniensis grew well under regular culture condition. However, because of its rarity in the clinical setting, conventional biochemical methods misidentified the bacterium as S. paucimobilis. WGS is a powerful molecular method to provide the definitive identification.
Most interestingly, the bacteria appeared to have originated from seawater. Sanmen Bay has muddy beaches with shallow seawater and high microbial richness suitable for marine aquaculture (http://www.sanmen.gov.cn/art/2018/6/5/art_1519452_20483713.html). F. hispaniensis also probably lives in seawater and under the right conditions could cause human infections. In 1 F. hispaniensis case, a woman in Australia had a fishhook injury, which was consistent with the seawater exposure hypothesis (4). Other Fransicella species, such as F. noatunensis, which inhabits the ocean, are major pathogens for fish and shellfish (10). The patient in our report acquired infection in August, the hottest month in Zhejiang Province. The high temperature could promote bacteria growth in the seawater and increase the likelihood of human exposure.
The F. hispaniensis isolate in our report exhibited a similar antimicrobial susceptibility pattern to F. tularensis. This finding is consistent with a study showing susceptibility of all 91 Francisella strains tested to aminoglycosides, tetracycline, and fluoroquinolones (11). Fluroquinolones, such as ciprofloxacin, are highly effective in treating infections caused by F. tularensis (12), F. philomiragia (2,13), F. novicida (14), and F. hispanensis (4). Third-generation cephalosporins and carbapenems are generally not active against Francisella spp. (9,11), as shown by failed treatment with ceftizoxime and imipenem in the case we describe. Studies based on mouse models showed moxifloxacin is more effective than ciprofloxacin in treating tularemia and is less affected by treatment delay (9,15). In this patient, moxifloxacin successfully treated F. hispaniensis infections without relapse.
In summary, clinicians need to be aware of the emerging and highly pathogenic F. hispaniensis, which is resistant to many β-lactams, including the cephalosporins and carbarpenems commonly used for empirical treatment. Our report also demonstrates that seawater exposure can be a risk factor for acquiring F. hispaniensis infection.
Dr. Hua Zhou is a clinical physician and researcher at the Department of Respiratory and Critical Care Medicine, First Affiliated Hospital of Zhejiang University School of Medicine. His primary research interest is the diagnosis and treatment of respiratory tract infections and sepsis.
Acknowledgments
We thank Bin Hu and Jiale Zhou for performing the metagenomic sequencing test and WGS tests.
This work was supported by a research grant from the National Natural Science Foundation of China (81971897). The research funding was provided to H.Z.
References
- Maurin M, Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect Dis. 2016;16:113–24. DOIPubMedGoogle Scholar
- Kreitmann L, Terriou L, Launay D, Caspar Y, Courcol R, Maurin M, et al. Disseminated infection caused by Francisella philomiragia, France, 2014. Emerg Infect Dis. 2015;21:2260–1. DOIPubMedGoogle Scholar
- Huber B, Escudero R, Busse HJ, Seibold E, Scholz HC, Anda P, et al. Description of Francisella hispaniensis sp. nov., isolated from human blood, reclassification of Francisella novicida (Larson et al. 1955) Olsufiev et al. 1959 as Francisella tularensis subsp. novicida comb. nov. and emended description of the genus Francisella. Int J Syst Evol Microbiol. 2010;60:1887–96. DOIPubMedGoogle Scholar
- Aravena-Román M, Merritt A, Inglis TJ. First case of Francisella bacteraemia in Western Australia. New Microbes New Infect. 2015;8:75–7. DOIPubMedGoogle Scholar
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-ninth informational supplement (M100-S29). Wayne (PA): The Institute; 2019.
- Bina XR, Wang C, Miller MA, Bina JE. The Bla2 beta-lactamase from the live-vaccine strain of Francisella tularensis encodes a functional protein that is only active against penicillin-class beta-lactam antibiotics. Arch Microbiol. 2006;186:219–28. DOIPubMedGoogle Scholar
- Antunes NT, Frase H, Toth M, Vakulenko SB. The class A β-lactamase FTU-1 is native to Francisella tularensis. Antimicrob Agents Chemother. 2012;56:666–71. DOIPubMedGoogle Scholar
- Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15:631–46. DOIPubMedGoogle Scholar
- Caspar Y, Maurin M. Francisella tularensis susceptibility to antibiotics: a comprehensive review of the data obtained in vitro and in animal models. Front Cell Infect Microbiol. 2017;7:122. DOIPubMedGoogle Scholar
- Birkbeck TH, Feist SW, Verner-Jeffreys DW. Francisella infections in fish and shellfish. J Fish Dis. 2011;34:173–87. DOIPubMedGoogle Scholar
- Georgi E, Schacht E, Scholz HC, Splettstoesser WD. Standardized broth microdilution antimicrobial susceptibility testing of Francisella tularensis subsp. holarctica strains from Europe and rare Francisella species. J Antimicrob Chemother. 2012;67:2429–33. DOIPubMedGoogle Scholar
- Boisset S, Caspar Y, Sutera V, Maurin M. New therapeutic approaches for treatment of tularaemia: a review. Front Cell Infect Microbiol. 2014;4:40. DOIPubMedGoogle Scholar
- Mailman TL, Schmidt MH. Francisella philomiragia adenitis and pulmonary nodules in a child with chronic granulomatous disease. Can J Infect Dis Med Microbiol. 2005;16:245–8. DOIPubMedGoogle Scholar
- Brett ME, Respicio-Kingry LB, Yendell S, Ratard R, Hand J, Balsamo G, et al. Outbreak of Francisella novicida bacteremia among inmates at a louisiana correctional facility. Clin Infect Dis. 2014;59:826–33. DOIPubMedGoogle Scholar
- Piercy T, Steward J, Lever MS, Brooks TJ. In vivo efficacy of fluoroquinolones against systemic tularaemia infection in mice. J Antimicrob Chemother. 2005;56:1069–73. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: September 02, 2020
1These authors contributed equally to this article.
Table of Contents – Volume 26, Number 10—October 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jianying Zhou, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Zhejiang University School of Medicine, 79 Qingchun Rd, Hangzhou, Zhejiang, China; ; Shangxin Yang, UCLA Clinical Microbiology Laboratory, 11633 San Vicente Blvd., Los Angeles, CA 90049, USA
Top