Volume 26, Number 12—December 2020
Synopsis
Control and Prevention of Anthrax, Texas, USA, 2019
Abstract
The zoonotic disease anthrax is endemic to most continents. It is a disease of herbivores that incidentally infects humans through contact with animals that are ill or have died from anthrax or through contact with Bacillus anthracis–contaminated byproducts. In the United States, human risk is primarily associated with handling carcasses of hoofstock that have died of anthrax; the primary risk for herbivores is ingestion of B. anthracis spores, which can persist in suitable alkaline soils in a corridor from Texas through Montana. The last known naturally occurring human case of cutaneous anthrax associated with livestock exposure in the United States was reported from South Dakota in 2002. Texas experienced an increase of animal cases in 2019 and consequently higher than usual human risk. We describe the animal outbreak that occurred in southwest Texas beginning in June 2019 and an associated human case. Primary prevention in humans is achieved through control of animal anthrax.
The zoonotic disease anthrax, caused by the bacterium Bacillus anthracis, has been known to humankind for thousands of years and is endemic to most continents (1–3). It is a naturally occurring disease of herbivores that incidentally infects humans through contact with animals that are ill or have died from anthrax or through contact with B. anthracis–contaminated byproducts such as meat, hides, hair, and wool (4). Transmission routes include cutaneous, ingestion, inhalation, and injection; cutaneous accounts for most (95%) cases worldwide (2,4). In the United States, human risk is primarily associated with handling carcasses of hoofstock that have died of anthrax; the primary risk for herbivores is ingestion of B. anthracis spores that can persist in suitable alkaline soils in a corridor from Texas through Colorado, the Dakotas, and Montana (5–7).
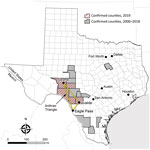
The 2 state agencies responsible for anthrax surveillance in Texas are the Texas Department of State Health Services (DSHS) and the Texas Animal Health Commission (TAHC). Samples that are culture-positive for B. anthracis at veterinary reference laboratories are reported to DSHS and TAHC. Veterinarians treating animals with illnesses compatible with anthrax must also report to DSHS and TAHC. Suspected cases of human anthrax are immediately reportable to DSHS. Samples or isolates from human cases are forwarded for identification to local public health reference laboratories. In Texas, animal anthrax cases are most commonly reported from the triangular area bounded by the towns of Uvalde, Ozona, and Eagle Pass (Figure 1), which includes portions of Crockett, Val Verde, Sutton, Edwards, Kinney, Uvalde, Zavala, and Maverick Counties in southwestern Texas.
During 2000–2018, a total of 63 animal anthrax cases were confirmed by culture of B. anthracis in a reference laboratory (annual mean 3.3, range 0–20 cases/year) (T. Sidwa, unpub. data). Because only 1 animal per affected premise usually is reported in a given year, the number of cases is a substantial underrepresention of the total number of affected animals and properties. The last naturally occurring human case of cutaneous anthrax associated with livestock exposure in Texas was reported in 2001 (8,9).
Animal Cases
Texas Veterinary Medical Diagnostic Laboratory confirmed the first anthrax case of 2019 in an exotic antelope carcass from Uvalde County on June 19. Overall in 2019, the laboratory reported 25 culture-positive animals, including cattle, horses, white-tailed deer, antelope, and a goat, from Crockett, Kinney, Sutton, Uvalde, and Val Verde counties. The last confirmed animal case was reported on August 21. Unconfirmed numbers reported to DSHS staff suggest that >1,000 animal losses might be attributed to the 2019 outbreak (K. Waldrup, unpub. data).
Implementing control measures (i.e., vaccination and proper carcass disposal) was challenging; thin topsoil over bedrock, vast and inaccessible terrain, and burn bans triggered by hot, dry weather conditions made it difficult for livestock owners and landowners to identify and bury or burn dead animals. Livestock owners can sometimes cover dead animals with tarps if burial or burning is not an option. However, because properties in this area of Texas can be thousands of acres and not particularly navigable, reaching dead animals to cover and protect them from scavengers (that might further distribute B. anthracis–contaminated remains) is often not feasible.
Another obstacle to controlling the outbreak was the inability to address the contribution of wildlife to the initiation and perpetuation of disease spread (e.g., lack of a licensed vaccine and impracticality of using physical or chemical restraint to administer vaccine “off label” to wildlife species). In addition, reports of vaccine-associated adverse events among goats and horses (2,10) made some owners reluctant to vaccinate these species. Among confirmed animal anthrax cases in species for which vaccination is indicated (cattle, goats, horses, sheep, and swine) (11), a third are reported to have been vaccinated before illness. Of those, the median number of days from most recent vaccination to specimen collection was 8 days (range 3–82 days) (T. Sidwa, unpub. data). The frequency and effect of antibiotic use subsequent or simultaneous to vaccination was unknown.
Human Case Report
On July 23, 2019, a non-Hispanic White man in his 70s from the anthrax-affected area who had a history of cardiovascular disease and hypertension visited his physician for evaluation of 2 lesions near his right knee. Four days earlier, a small red spot had emerged and gradually enlarged and became painful. He reported no fever and used no over-the-counter medications. When asked about animal exposures because of where he lived, he reported that he and his daughter had moved 2 fly-covered deer carcasses from beneath his porch before lesion onset. He was wearing shorts and a shirt while moving the carcasses, and his affected leg was scraped by the velvet-covered antlers. He also reported being bitten by a fly. The deer carcasses were not tested for anthrax, and the patient disposed of them.
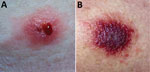
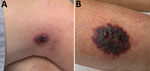
On examination at his physician’s office, the patient’s vital signs were as follows: blood pressure 177/87 mm Hg; heart rate 76 beats/min; and temperature 98.3°F. Below and lateral to his right knee was an indurated, raised, erythematous 5-cm lesion with small ulcerations that oozed serosanguinous fluid and was surrounded by a blanched halo. Just proximal to his right knee was a nonindurated erythematous macule (Figure 2). No popliteal or inguinal adenopathy was present. After 2 swab specimens were obtained from the larger lesion, the patient was given a cephalosporin intramuscularly, and a prescription for ciprofloxacin was called in to his pharmacy of choice more than an hour’s drive from his home. Because it was too late to send the specimens anywhere for testing on that day, the swabs were mailed directly to the Texas Department of State Health Services Laboratory on Wednesday after a phone consultation with the state health department.
The patient began his ciprofloxacin the next evening (July 24). On July 26, after having taken 4 doses of his antibiotics, he was feeling worse and sought additional care at the emergency department of hospital A, more than an hour’s drive from his residence. Concurrently, the state laboratory notified his primary-care physician that a preliminary laboratory report for the specimen was PCR-positive for B. anthracis; this result was confirmed by culture the following week (August 1) (Figures 3, 4). His physician relayed the information first to the patient and then to hospital staff. Upon arrival to the hospital, the patient reported pain, difficulty walking, and nausea. He reported intermittent spontaneous drainage of a dark, jelly-like material from the larger wound. He reported no fever, chills, chest pain, shortness of breath, pain at rest, numbness, or tingling. He did not use tobacco products.
At hospital A, he reported that his exposure had been »3 weeks earlier. At examination, his vital signs were blood pressure 132/71 mm Hg; heart rate 91 beats/min; and respirations 24 breaths/min. He was afebrile. He had a nondraining, nonerythematous eschar 7.2 cm × 5 cm on the lateral aspect of the right calf and a painless, nondraining, nonerythematous 3.3 cm × 2 cm eschar on the lateral aspect of the right knee (Figure 5). His leukocyte count was 12,000 (103 cells/µL); hemoglobin, 15.5 g/dL; hematocrit, 46.9% g/dL; platelets, 83,000 (103 cells/mL); blood urea nitrogen, 35 mg/dL; and creatinine, 2.6 mg/dL. His antibiotic was switched to intravenous doxycycline (100 mg every 12 hours). He was discharged on hospital day 13.
Control Measures for Animal Outbreaks
Because naturally occurring human anthrax cases in endemic countries are almost always related to exposure to infected animals or their byproducts, control of animal anthrax essentially eliminates human risk. The primary control measure for animal anthrax is annual preventive vaccination; however, once an outbreak occurs, other control measures include ring vaccination, proper carcass disposal to avoid further environmental contamination, and quarantine (i.e., limit animal movement from the affected and nearby properties, animal contact with anthrax-contaminated sites, and contact between affected and nonaffected herds) (9). On the basis of anecdotal reports and 1 small study, tabanid flies (e.g., deer and horse flies) might play a role in transmission; whether fly control is achievable or would be effective remains an open question (2,12,13).
The attenuated Sterne-strain of B. anthracis is used globally for vaccination among domestic livestock (14). Because the vaccine is live-attenuated, concurrent antibiotic administration can substantially diminish efficacy. If an animal is given antibiotics either 10 days before or after vaccination, revaccination is recommended (9,15). Whether concurrent administration of antibiotics played a role in diminished vaccine efficacy in the Texas outbreak is unclear.
Proper and safe carcass disposal is critical for controlling anthrax outbreaks in enzootic areas because inappropriate carcass disposal seeds the soil with spores and increases the risk for future epizootics. Global recommendations (9) and codified Texas regulations (16) for carcass disposal are similar: the carcass should be burned in place, using a pyre or other method that leaves only ash and allows the destruction of the contaminated soil as well (i.e., “burnt until it is thoroughly consumed”) (9,16). When a carcass cannot be burned, global recommendations are to bury it deeply (9). The historic practice of adding lime should be avoided (17). High soil calcium levels, either from the addition of lime or as occur naturally in southwest Texas, are conducive to B. anthracis spore survival (6,7) and increase the likelihood of future outbreaks. The least desirable disposal method is leaving the carcass in place, because scavenging can further disseminate the spores and increase future exposure risks for susceptible animals. Alternative carcass disposal methods are needed in areas where the standard recommendations to burn or bury carcasses are impractical. This need is particularly pronounced where there is an abundance of susceptible wildlife species that are not vaccinated or where there is poor vaccination coverage of domestic hoofstock.
Prevention of Human Cases in Endemic Areas
Human and animal health authorities should remind at-risk populations of the following prevention measures when animal cases are first identified. During animal outbreaks of anthrax, persons who handle and dispose of infected animals are at highest risk for exposure. However, exposure can be minimized through use of personal protective equipment, which should include gloves that can be disinfected or disposed of, long sleeves and pants, and footwear suitable to the terrain that can be disinfected (9). Even in the absence of a recognized anthrax outbreak, veterinarians and ranchers in endemic areas should always keep anthrax in mind as they interact with members of susceptible species that are ill. To do otherwise can result in inadvertent exposure to anthrax.
Antibiotic postexposure prophylaxis (PEP) is another important component of prevention. In the former Soviet Union, before 1965, 58/339 (17%) of patients who did not receive antibiotic prophylaxis after cutaneous exposures had onset of anthrax; in contrast, only 5/287 (2%) who received prophylaxis had onset of anthrax (18).
If skin or mucus membrane contact occurs during carcass disposal, persons should seek medical attention and receive antibiotic PEP for 7 days (Table 1) and have their symptoms monitored for 14 days. Although aerosol exposure is unlikely in cases of natural cutaneous exposures, if potential aerosol exposure also occurred, antibiotic PEP should be administered for up to 60 days and anthrax vaccine may be considered.
Persons who live and work in anthrax-endemic areas and who anticipate interacting with animals that are dying or have died of anthrax might wish to consider preexposure prophylaxis with anthrax vaccine adsorbed (AVA). For preexposure prophylaxis of persons at high risk for B. anthracis exposure, AVA is administered intramuscularly as a priming series at 0, 1, and 6 months, with booster doses at 12 and 18 months and annually thereafter (19). Health departments in endemic areas that have existing vaccination programs can acquire AVA from the manufacturer.
Healthcare Infection Control Issues for Cutaneous Anthrax
A person with cutaneous or other type of anthrax (e.g., injection, ingestion, or inhalation) cannot transmit disease through aerosol or droplet. However, spores that could remain on a person’s skin, hair, or clothing after an exposure before they bathe or shower and change clothes might possibly transfer to someone else’s skin and cause cutaneous anthrax (20–22). Although incubation periods of <1 day are reported, patients usually wait a few days to seek care, making it likely that they would already have bathed and changed clothes before seeking care. It is therefore unlikely that healthcare personnel would be secondarily exposed to spores.
Although cutaneous anthrax lesions can be contagious before the institution of effective antibiotic therapy, they become sterile in <1 day once therapy has begun (23). Lesions should be covered until the patient has had 24 hours of effective antibiotics. Contact precautions should be used for the first day; after that, standard precautions are sufficient.
Disposable items that have been in direct contact with the anthrax lesion, any tissue removed during debridement, and potentially infectious wound care materials (24,25) should be disposed of in a biohazard bag according to guidelines for disposal of any potentially infectious material. No additional disinfection is needed beyond what is regularly scheduled for the facility. Nondisposable surfaces in direct contact with the anthrax lesion or wound drainage can be disinfected with a 0.5% hypochlorite solution, a commercial product such as SporGon (Decon Labs, https://deconlabs.com), or other sporicidal agents such as an Environmental Protection Agency–registered antimicrobial product effective against B. anthracis spores (26–28); products effective against Clostridium difficile spores might also be appropriate (29,30)
Diagnosis
Although an eschar is the cardinal sign of cutaneous anthrax, in its early stages, anthrax can manifest as a group of small vesicles that might be pruritic. The lesion might be surrounded by erythema and swelling but is usually painless. Lymphadenopathy can occur, and constitutional symptoms including fever and headache are also possible. Localized cutaneous anthrax can disseminate to become a systemic disease. Although a substantial portion (10%–40%) of patients with cutaneous anthrax would die if left untreated (4), most can recover with treatment (31). Meningitis is also a possible, and typically fatal, complication (32).
In the United States, cutaneous anthrax is decidedly rare: other causes of eschars and eschar-like lesions include poxvirus infections (e.g., cowpox, vaccinia, orf), rickettsial infections (e.g., scrub typhus and Rickettsia parkeri rickettsiosis), ulceroglandular tularemia, staphylococcal or streptococcal infections, and noninfectious causes such as insect or spider bites. Obtaining a good exposure history is key to determining the likelihood of various etiologies among the differential diagnoses and determining the best specimens to collect. Patients seeking care with an eschar or eschar-like lesion should be asked about recent exposure to dead or dying herbivores or biting flies in an anthrax enzootic area; recent animal bites or scratches; and recent contact with lagomorphs, rodents, fleas, ticks, and spiders.
A Gram stain of a swab specimen from the lesion can often quickly identify possible cases and narrow the differential diagnosis (23). Specimens for tests such as Gram stain, culture, and PCR to rule anthrax in or out (Table 2) must be collected before the use of antibiotic therapy because they will rapidly become negative after the implementation of therapy (23). Specimens can be sent to sentinel laboratories for preliminary assessment. Specimens for which B. anthracis is not ruled out by a sentinel laboratory should promptly be sent to a Laboratory Response Network (LRN) laboratory for confirmation (33). LRN is a network of laboratories established to respond to biologic and chemical threats and other public health emergencies that consists of 3 types of laboratories. Private and commercial laboratories comprise the first tier of the LRN and are described as sentinel laboratories. Laboratories that receive reagents, protocols, and specialized training to perform confirmatory testing for multiple agents in high-risk environmental or clinical samples comprise the second tier of LRN and are referred to as reference laboratories. Specialized characterization of organisms, bioforensics, select agent activity, and handling of highly infectious biologic agents is performed at national laboratories, the third tier of LRN. However, with approval from public health authorities, specimens from lesions that are highly suspicious based on clinical or epidemiologic grounds can be sent directly from clinicians to an LRN laboratory (34).
Notification
Clinicians should promptly notify their local or state health department when they suspect anthrax, although the mandated timing varies by jurisdiction. State and territorial health departments should notify the Centers for Disease Control and Prevention (CDC) within 4–24 hours (24) of the initial report for patients whose illness meets the probable or confirmed case definition (35). Presumptive positive results from an LRN laboratory must be reported within 2 hours to the state and CDC.
Treatment
Cutaneous anthrax lacking systemic manifestations such as fever, tachycardia, tachypnea, hypotension, leukocytosis, or leukopenia can usually be treated with 7 days of an oral antibiotic. Patients with cutaneous anthrax should only continue oral antibiotics for PEP after antibiotic treatment is complete if the patient was also exposed to aerosolized spores; this would rarely be indicated for naturally acquired cutaneous infections because aerosol exposures are unlikely (Table 1).
Systemically ill patients should be evaluated for meningitis; if meningitis can be ruled out, they should be treated with at least 2 intravenous antibiotics (1 that is bactericidal and 1 that inhibits protein synthesis to block toxin production). Antibiotic therapy should continue for >2 weeks or until the patient is stable. If meningitis is present, >3 antibiotics should be used (>1 should be bactericidal, >1 should inhibit protein synthesis, and all should have good central nervous system penetration). Antibiotic options for treatment and prevention of anthrax are listed in Tables 1 and 3.
Systemically ill patients (whether from cutaneous, ingestion, inhalation, or injection exposures) are candidates for 1 of the Food and Drug Administration–approved anthrax antitoxins. The antitoxins are available through the Strategic National Stockpile pending a consultation with an anthrax subject matter expert at CDC, which can be reached by calling the Emergency Operations Center (770-488-7100).
Surgery might occasionally be indicated for lesions complicated by compartment syndrome. However, surgery usually is not necessary for cutaneous anthrax (36).
Anthrax is endemic to parts of the United States. Epizootics emerge with varying frequency when climatic conditions favor the uncovering of soilborne B. anthracis spores with subsequent consumption by susceptible herbivores. Humans contract cutaneous anthrax through contact with animals that are ill or have died from anthrax or contact with B. anthracis–contaminated byproducts; this risk is increased during epizootics. The outbreak we describe was confirmed in June 2019, but its actual start date is unknown; reliable recognition of epizootics might be impeded when they occur in vast, rough, and sparsely populated areas such as those in the anthrax-endemic areas of Texas. These same geographic characteristics create challenges in implementing the recommended disease control interventions, including appropriate carcass disposal and broad use of animal anthrax vaccine in species for which the vaccine is licensed, as well as off-label use in other species. Wild herbivores (e.g., white-tailed deer and exotic hoofstock) contributed to the 2019 Texas outbreak, but effective mitigation (carcass disposal or vaccination) of the risk they posed could not be adequately achieved.
The cutaneous anthrax patient associated with this outbreak was apparently exposed through a scratch on the leg from the antler of an untested deer carcass. The physician he visited in rural Texas included anthrax in the differential diagnosis, obtained and submitted diagnostic samples before treating the patient, and provided the patient with a prescription for oral ciprofloxacin. Anthrax was identified through PCR and confirmed through culture at the state reference laboratory from swab specimens of a leg lesion. The patient was treated as an outpatient with appropriate antibiotics until his condition worsened and required a 13-day hospitalization. The necessity for hospitalization might have been related to a few-week delay in seeking treatment. Despite the delay, the patient, like most patients with cutaneous anthrax, survived with antibiotic treatment (4,32).
As soon as anthrax is recognized in an animal population, public health and animal health agencies must collaborate to heighten awareness among medical and animal health communities, as well as among ranchers and other inhabitants of at-risk areas. Timely delivery of information to ranchers on proper carcass disposal and appropriate use of personal protective equipment, as was done through various alerts, might reduce the number of exposures. If exposure is recognized, antibiotic PEP should be considered by medical providers. AVA may be appropriate for persons at high risk for exposure, such as veterinary staff and ranch workers in endemic areas; however, this process involves a long-term commitment to annual booster shots to ensure protection.
Ranchers and veterinarians should receive authoritative information on animal vaccine use to break the cycle of transmission (including emphasis on avoiding administration of antibiotics 10 days before or after vaccine administration). Even in the absence of a recognized anthrax outbreak, veterinarians and ranchers in endemic areas should keep anthrax in mind as they interact with ill members of susceptible species. Doing otherwise might result in inadvertent exposure to anthrax. A survey of ranchers in the outbreak area is planned by TAHC to assess knowledge, attitudes, and practices regarding anthrax, including information on livestock vaccination.
Recent federal anthrax guidance has focused on the treatment of systemic anthrax, including meningitis, rather than on the more common cutaneous form of the disease. Given that half the cases in the 2001 anthrax incident in the United States (37) were cutaneous anthrax and most sporadic cases in the United States and worldwide are cutaneous, this article provides an overview of prevention and control measures for animals and a single resource for the prevention, diagnosis, infection control, and treatment of naturally acquired cutaneous anthrax.
Dr. Sidwa worked for the Texas Department of State Health Services from 1993 to 2020 (retired January 31, 2020), most recently managing the Zoonosis Control Branch and serving as the State Public Health Veterinarian.
Acknowledgments
We thank Amanda Kieffer, Trevor Maness, Amanda Kammen, Kelly Persinger, Laura Robinson, Kelly Broussard, and Bonny Mayes for their assistance with this investigation and contributions to this article. We would also like to thank Thitipong Mongkolrattanothai for assistance with references and Mark Deka for preparing a map of the affected area.
This work is in memory of Robert “Dr. Bob” Dittmar, a friend, colleague, and Texas Parks and Wildlife Department (TPWD) State Wildlife Veterinarian who, along with 2 TPWD colleagues, was killed August 8, 2020, in a helicopter crash while conducting aerial surveys for desert bighorn sheep in TPWD’s Black Gap Wildlife Management Area. He is missed.
References
- Fasanella A, Galante D, Garofolo G, Jones MH. Anthrax undervalued zoonosis. Vet Microbiol. 2010;140:318–31. DOIPubMedGoogle Scholar
- Shadomy SV, Smith TL. Zoonosis update. Anthrax. J Am Vet Med Assoc. 2008;233:63–72. DOIPubMedGoogle Scholar
- Doganay M, Metan G, Alp E. A review of cutaneous anthrax and its outcome. J Infect Public Health. 2010;3:98–105. DOIPubMedGoogle Scholar
- Griffin DW, Silvestri EE, Bowling CY, Boe T, Smith DB, Nichols TL. Anthrax and the geochemistry of soils in the contiguous United States. Geosciences (Basel). 2014;2014:114–27. DOIGoogle Scholar
- Blackburn JK, McNyset KM, Curtis A, Hugh-Jones ME. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecological [corrected] niche modeling. Am J Trop Med Hyg. 2007;77:1103–10. DOIPubMedGoogle Scholar
- Yang A, Mullins JC, Van Ert M, Bowen RA, Hadfield TL, Blackburn JK. Predicting the geographic distribution of the Bacillus anthracis A1.a/Western North American sub-lineage for the continental United States: new outbreaks, new genotypes, and new climate data. Am J Trop Med Hyg. 2020;102:392–402. DOIPubMedGoogle Scholar
- Barnes S, Tull C, Neidert M. Confirmed human cutaneous anthrax in PHR 8. Texas Disease Prevention News. 2001;61:1–4.
- World Health Organization. Anthrax in humans and animals. 4th ed. 2008 [cited 2020 Jan 31]. https://www.who.int/csr/resources/publications/AnthraxGuidelines2008
- Wobeser BK. Anthrax vaccine associated deaths in miniature horses. Can Vet J. 2015;56:359–60.PubMedGoogle Scholar
- World Organization for Animal Health (OIE). Anthrax manual of diagnostic tests and vaccines for terrestrial animals. 8th edition; 2018. p. 307–20.
- Turell MJ, Knudson GB. Mechanical transmission of Bacillus anthracis by stable flies (Stomoxys calcitrans) and mosquitoes (Aedes aegypti and Aedes taeniorhynchus). Infect Immun. 1987;55:1859–61. DOIPubMedGoogle Scholar
- Blackburn JK, Van Ert M, Mullins JC, Hadfield TL, Hugh-Jones ME. The necrophagous fly anthrax transmission pathway: empirical and genetic evidence from wildlife epizootics. Vector Borne Zoonotic Dis. 2014;14:576–83. DOIPubMedGoogle Scholar
- Hugh-Jones M. Overview of Anthrax. Merck veterinary manual 2015 [cited 2020 Aug 12]. https://www.merckvetmanual.com/generalized-conditions/anthrax/overview-of-anthrax
- Forshaw D, Higgs AR, Moir DC, Ellis TM, Links IJ. Anthrax in cattle in southern Western Australia. Aust Vet J. 1996;74:391–3. DOIPubMedGoogle Scholar
- Texas Legal Code. Anthrax disposal [chapter 31]. §313 adopted to be effective December 18, 1992, 17 TexReg 8286; amended to be effective April 4, 1999, 24 TexReg 2298 1999 [cited 2020 Jan 31]. http://txrules.elaws.us/rule/title4_chapter31_sec.31.3
- Himsworth CG. The danger of lime use in agricultural anthrax disinfection procedures: the potential role of calcium in the preservation of anthrax spores. Can Vet J. 2008;49:1208–10.PubMedGoogle Scholar
- Kebedzhiev G. [Prophylaxis of anthrax by antibiotics] [in Russian]. Antibiotiki. 1970;15:89–93.PubMedGoogle Scholar
- Bower WA, Schiffer J, Atmar RL, Keitel WA, Friedlander AM, Liu L, et al.; ACIP Anthrax Vaccine Work Group. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices, 2019. MMWR Recomm Rep. 2019;68:1–14. DOIPubMedGoogle Scholar
- Clarke PS. Chloramphenicol in treatment of cutaneous anthrax. BMJ. 1952;1:86–7. DOIPubMedGoogle Scholar
- Amidi S, Dutz W, Kohout E, Ronaghy A. Human anthrax in Iran. Report of 300 cases and review of literature. Tropenmed Parasitol. 1974;25:96–104.PubMedGoogle Scholar
- Reilly WA, Beeson CR. Antibiotic therapy for cutaneous anthrax: report of five cases. Arch Intern Med (Chic). 1948;82:529. DOIGoogle Scholar
- Gaitanis G, Lolis CJ, Tsartsarakis A, Kalogeropoulos C, Leveidiotou-Stefanou S, Bartzokas A, et al. An aggregate of four anthrax cases during the dry summer of 2011 in Epirus, Greece. Dermatology. 2016;232:112–6. DOIPubMedGoogle Scholar
- Heninger SJ, Anderson CA, Beltz G, Onderdonk AB. Decontamination of Bacillus anthracis spores: evaluation of various disinfectants. Appl Biosaf. 2009;14:7–10. DOIPubMedGoogle Scholar
- Evers DL, Allen KP, Fowler CB, Mason JT, Blacksell SD. Laboratory decontamination of HHS-listed and HHS/USDA overlap select agents and toxins. Appl Biosaf. 2013;18:59–72. DOIGoogle Scholar
- Oudejans L, Mickelsen L, McConkey K. Effectiveness of disinfecting and sporicidal wipes against Bacillus atrophaeus, a Bacillus anthracis surrogate. 2017 [cited 2020 Aug 12]. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHSRC&dirEntryId=337359
- US Environmental Protection Agency. List K: EPA’s registered antimicrobial products effective against Clostridium difficile spores. 2016 [cited 2020 Aug 12]. https://19january2017snapshot.epa.gov/sites/production/files/2016-06/documents/list_k_clostridium.pdf
- US Environmental Protection Agency. Comparative efficacy of sporicidal technologies for the decontamination of Bacillus anthracis, B. atrophaeus, and Clostridium difficile spores on building materials. 2015 [cited 2020 Aug 12]. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHSRC&dirEntryId=303850
- Davies JC. A major epidemic of anthrax in Zimbabwe. The experience at the Beatrice Road Infectious Diseases Hospital, Harare. Cent Afr J Med. 1985;31:176–80.PubMedGoogle Scholar
- American Society for Microbiology. Sentinel level clinical laboratory guidelines for suspected agents of bioterrorism and emerging infectious diseases. Bacillus anthracis and Bacillus cereus biovar anthracis. 2017 [cited 2020 Aug 12]. https://www.asm.org/ASM/media/Policy-and-Advocacy/LRN/Sentinel%20Files/AnthraxLRN-Aug2017.pdf
- Centers For Disease Control and Prevention. The Laboratory Response Network: partners in preparedness. 2019 [cited 2020 Aug 12]. https://emergency.cdc.gov/lrn
- Centers For Disease Control and Prevention. National Notifiable Diseases Surveillance System: anthrax 2018 case definition. 2018 [cited 2020 Aug 12]. https://wwwn.cdc.gov/nndss/conditions/anthrax/case-definition/2018
- Doganay M. International medicine: major tropical syndromes: systemic infections [section 6]. In: Cohen J, Powderly WG, Opal SM, editors. Infectious diseases. 4th ed: Elsevier; 2017. p. 1123–8.
- Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, et al.; National Anthrax Epidemiologic Investigation Team. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8:1019–28. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: November 12, 2020
Table of Contents – Volume 26, Number 12—December 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Kate Hendricks, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H24-12, Atlanta, GA 30329-4027, USA
Top