Volume 26, Number 2—February 2020
Dispatch
Systematic Hospital-Based Travel Screening to Assess Exposure to Zika Virus1
Abstract
We queried hospital patients about international travel in the previous 30 days to assess potential importation of emerging infections. We used 12 months of deidentified data to analyze patient demographics, travel destinations, and diagnoses for exposure to Zika virus. Our approach could be used to analyze potential infectious disease exposures.
Incidence of Zika virus (ZIKV) infections rose rapidly in early 2015, and local transmission was confirmed in 84 countries and territories by March 2017 (1). Although ZIKV typically causes mild symptoms (2,3), in utero infection can cause congenital Zika syndrome (4,5). The threat of in utero infection, along with sexual transmission (6,7), led to advisories for women who were pregnant, or might become pregnant, and their partners to avoid travel to countries or areas with ZIKV transmission (7–10).
After implementing reactive screening during several global infectious disease outbreaks, including the 2014 Ebola outbreak, Mount Auburn Hospital (Cambridge, Massachusetts, USA) incorporated a standardized screening question regarding international travel into all hospital visits beginning in September 2015. To detect potential travel-associated exposures, patients were asked, “Have you traveled outside of the U.S. within the past 30 days?” Each quarter during November 1, 2015–October 31, 2016, we aggregated deidentified patient data to estimate the proportion of patients with potential ZIKV exposure and the possibility for congenital Zika syndrome.
During November 1–December 31, 2016, we retrospectively analyzed deidentified patient demographic, travel destination, and medical services data from the hospital database. We analyzed records from patients admitted as inpatients, and those seen in the emergency department/walk-in center (ED/WIC) and by other services during November 1, 2015–October 31, 2016. We included data from patients who responded “yes” to the travel screening question and provided a travel history and for whom diagnostic data were available. We categorized destination countries according to the World Health Organization 2016 classification for ZIKV transmission (11): category 1, countries that reported outbreaks from 2015 onward; category 2, countries with possible endemic transmission or evidence of local mosquitoborne ZIKV infections in 2016; and category 3, countries with evidence of local mosquitoborne ZIKV infections during or before 2015, but without documentation of cases in 2016, or designated as outbreak terminated. We defined reproductive age as 15–49 years of age for female patients and ≥15 years of age for male patients (12). We extracted records with International Classification of Diseases, 10th Revision, codes applicable to pregnancy, including Z33.1, Z34.91, Z34.92, Z34.93, and Z34.90, and diagnosis descriptions that met the Zika disease case definition (3), which includes fever, rash, arthralgia, conjunctivitis, complication of pregnancy, or Guillain-Barré syndrome. We performed analyses by using IBM SPSS Statistics 17.0 (IBM, https://www.ibm.com). The Mount Auburn Hospital Institutional Review Board determined the activity to be exempt from review and approval.
We identified 5,642 patients who reported travel <30 days before their hospital visit. Of 5,004 patients who had complete demographic, destination, and diagnostic data, 3,109 (62.1%) were female and 1,895 (37.9%) male; patients were 10 months–94 years of age. A total of 959 (19%) were evaluated in the ED/WIC, and 161 (3.2%) with recent travel were hospitalized. The most frequently visited destinations were Canada, the United Kingdom, and Mexico (Table 1).
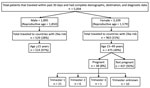
Among female patients, 1,579 (50.8%) were of reproductive age and 176 (5.7%) were pregnant. Among male patients, 1,850 (97.6%) were of reproductive age. Overall, 475 (30%) female and 514 (28%) male patients of reproductive age traveled to countries with ZIKV transmission. Of 176 pregnant women, 38 (22%) had traveled to countries with ZIKV transmission within the previous 30 days; 21 were in the first trimester (Figure).
When analyzed for destinations in WHO categories 1–3 (11), 1,492 travelers, 963 female and 529 male, visited 1,570 destinations with ZIKV transmission. Mexico, the Dominican Republic, Aruba, Brazil, and Costa Rica (Table 1) were the most frequently visited destinations, and the Caribbean was the most frequently visited region (n = 648).
Among patients who traveled to countries with ZIKV transmission whose admitting diagnosis or description was available, 42 listed symptoms compatible with ZIKV infection (Table 2). We did not identify Guillain-Barré syndrome or any complications of pregnancy among the 42 patients, but 2 had laboratory-confirmed ZIKV infection.
Our results approximate the Global Travel Epidemiology Network analysis of pretravel consultations (13), in which 28% of 22,736 travelers planned trips to ZIKV-affected countries and >75% were of reproductive age. Another study retrospectively reviewed 46 patients for possible ZIKV infection and found 17% had laboratory evidence of infection (14). Applying this seropositivity rate to our study, if testing been done, 7 patients with symptoms compatible with ZIKV clinical criteria might have had laboratory-confirmed ZIKV infection.
We found that a standardized question to screen for international travel provided a description of travel patterns for this patient community. Data from the 12-month period coincided with the rapid spread of ZIKV and revealed the sizable portion of patients who might have been exposed to ZIKV during travel. Population-based analysis of travel-related ZIKV exposure could provide estimates of at-risk populations and diagnostic testing needs, especially for pregnant women. The application is especially promising with newer electronic health record systems. However, limited testing capability might have underestimated the actual number of travel-associated cases (15), even when clinicians suspected ZIKV.
Our study had some limitations. Because we only reviewed the population at 1 hospital during a single 12-month period, our results might not be generalizable to the US population. Our analysis relied on recent travel to countries and territories that reported ZIKV transmission, but some travelers might have visited risk-free settings, such as locations at higher altitudes, resulting in overestimation of the number of possible infections. Infectious ZIKV has been detected in semen mainly <30 days after fever onset, but its presence in semen has been documented longer (10,15); therefore, the potential number of ZIKV infections might exceed our estimate because sexual partners could become infected. Also, we did not have information on whether the patients of reproductive age were sexually active, fertile, had pregnant partners, or were planning conception. We might have missed cases for the following reasons: we relied on diagnoses and diagnostic descriptions, but omission of symptoms in these fields might not represent truly absent symptoms; some infected persons might have been unaware of ZIKV and might not have sought medical evaluation; the incubation period of sexually transmitted ZIKV might be >30 days and patients might have become ill after being seen; we did not collect or record ZIKV infections identified after the study period; only patients strongly suspected of ZIKV were tested due to limited laboratory capacity; and the travel screening question would not have identified sexually transmitted ZIKV infection in a patient who had not traveled internationally.
We used a systematic travel screening question to analyze potential exposure to ZIKV in a hospital population. Because up to 80% of ZIKV infections are asymptomatic (2), we used travel to Zika-affected countries as a proxy for potential ZIKV exposure. In patients with international travel <30 days before seeking treatment, 31.4% visited countries with ZIKV transmission. Half of the female patients and most male patients were of reproductive age. In this population, 30% of female patients who were of reproductive age or pregnant reported travel with potential exposure to ZIKV; male patients similarly were affected. Despite severe restrictions on testing for ZIKV infection at the time of the study, our analysis demonstrated the ability to identify patients with clinical findings that fit the ZIKV case definition even if they were not tested. We also identified a large proportion of patients who should have received Zika pretravel counseling.
Analysis of the hospitalwide data for recent travel history provided a tool to assess the proportion of the population that might have been exposed to ZIKV. These data could inform population-based ZIKV vaccination needs in the future. In addition, systematic travel screening also could be applied to other imported emerging infections in the future.
Acknowledgments
The authors thank Mary Elizabeth Wilson for her advice and Andrew Gardner for his assistance on data download and aggregation.
This project is supported in part by the Mount Auburn Hospital Medical Staff Executive Committee Research Grant 2015.
Disclosures: L.H.C. reports past advisor fees from Shoreland, Inc., and past Data Safety Monitoring Board service for Valneva, Inc., outside the submitted work. Other authors report no financial relationship.
Dr. Iqbal currently is a primary care physician at Providence Community Health Centers, Providence, Rhode Island, USA. His primary research interest is in the health of globally mobile residents, including recent and first generation immigrants.
References
- World Health Organization. Situation report: Zika virus, microcephaly, Guillain-Barre syndrome, 10 March 2017 [cited 2017 Nov 13]. https://www.who.int/emergencies/zika-virus/situation-report/10-march-2017
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374:1552–63. DOIPubMedGoogle Scholar
- Council of State and Territorial Epidemiologists. Zika virus disease and congenital Zika virus infection interim case definition and addition to the nationally notifiable diseases list [cited 2016 Apr 22]. http://www.cste2.org/docs/Zika_Virus_Disease_and_Congenital_Zika_Virus_Infection_Interim.pdf
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects–reviewing the evidence for causality. N Engl J Med. 2016;374:1981–7. DOIPubMedGoogle Scholar
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016;375:1–4. DOIPubMedGoogle Scholar
- Hamer DH, Wilson ME, Jean J, Chen LH. Epidemiology, prevention, and potential future treatments of sexually transmitted Zika virus infection. Curr Infect Dis Rep. 2017;19:16. DOIPubMedGoogle Scholar
- World Health Organization. Prevention of sexual transmission of Zika virus: interim guidance update, 6 September 2016. WHO/ZIKV/MOC/16.1 Rev.3 [cited 2016 September 6]. https://www.who.int/csr/resources/publications/zika/sexual-transmission-prevention
- Petersen EE, Polen KND, Meaney-Delman D, Ellington SR, Oduyebo T, Cohn A, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure–United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:315–22. DOIPubMedGoogle Scholar
- Oduyebo T, Petersen EE, Rasmussen SA, Mead PS, Meaney-Delman D, Renquist CM, et al. Update: Interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure–United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:122–7. DOIPubMedGoogle Scholar
- Oster AM, Russell K, Stryker JE, Friedman A, Kachur RE, Petersen EE, et al. Update: Interim guidance for prevention of sexual transmission of Zika virus–United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:323–5. DOIPubMedGoogle Scholar
- World Health Organization. Situation report: Zika virus, microcephaly, Guillain-Barré syndrome, 3 November 2016 [cited 2017 Nov 13]. https://www.who.int/emergencies/zika-virus/situation-report/3-november-2016
- World Health Organization. Reproductive health indicators: guidelines for their generation, interpretation and analysis for global monitoring [cited 2017 Nov 13]. https://apps.who.int/iris/bitstream/handle/10665/43185/924156315X_eng.pdf
- Lammert S, Walker AT, Erskine S, Rao SR, Esposito DH, Ryan ET, et al. Characteristics of US travelers to Zika virus-affected countries in the Americas, March 2015–October 2016. Emerg Infect Dis. 2017;23:324–7. DOIPubMedGoogle Scholar
- Valle J, Eick SM, Fairley JK, Waggoner JJ, Goodman RA, Rosenberg E, et al. Evaluation of patients for Zika virus infection in a travel clinic in the southeast United States, 2016. South Med J. 2019;112:45–51. DOIPubMedGoogle Scholar
- Graciaa DS, Collins MH, Wu HM. Zika in 2018: advising travelers amid changing incidence. Ann Intern Med. 2018;169:337–8. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: January 15, 2020
1Preliminary results were presented at the 65th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Atlanta, Georgia, November 12–17, 2016.
Table of Contents – Volume 26, Number 2—February 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Lin H. Chen, Mount Auburn Hospital, Division of Infectious Diseases and Travel Medicine, 330 Mount Auburn St, Cambridge, MA 02138, USA
Top