Volume 27, Number 1—January 2021
Research
Post–13-Valent Pneumococcal Conjugate Vaccine Dynamics in Young Children of Serotypes Included in Candidate Extended-Spectrum Conjugate Vaccines
Figure 5
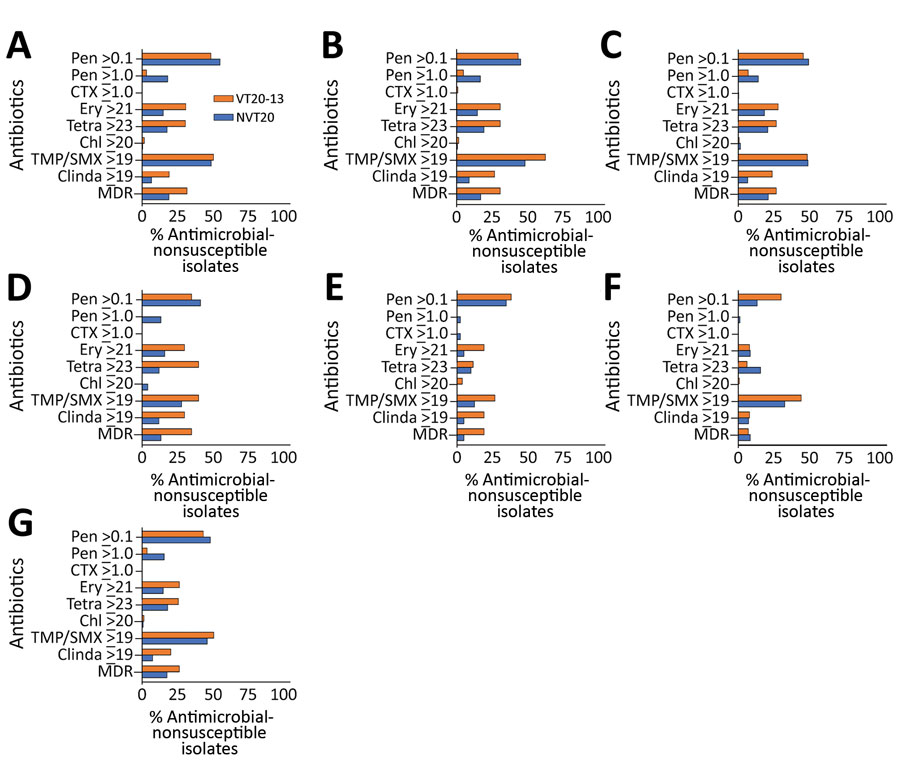
Figure 5. Proportion of antimicrobial drug–nonsusceptible isolates of all pneumococcal isolates in VT20–13 versus non-VT20 pneumococcal isolates in the late PCV13 period (2015–2017) in children <24 months of age in southern Israel. A) Healthy children; B) children with non–lower respiratory tract infections; C) children with lower respiratory tract infections; D) children with conjunctivitis; E) children with otitis media (isolates from middle ear fluid were tested); F) children with invasive pneumococcal disease; and G) all children. Chl, chloramphenicol; Clinda, clindamycin; CTX, ceftriaxone; Ery, erythromycin; MDR, multidrug resistance; PCV, pneumococcal conjugate vaccine; PCV13, 13-valent PCV; Pen, penicillin; Tetra, tetracycline; TMP/SMX, trimethoprim and sulphametoxazole; VT, vaccine serotype.