Limited Specificity of Serologic Tests for SARS-CoV-2 Antibody Detection, Benin
Anges Yadouleton
1, Anna-Lena Sander
1, Andres Moreira-Soto
1, Carine Tchibozo, Gildas Hounkanrin, Yvette Badou, Carlo Fischer, Nina Krause, Petas Akogbeto, Edmilson F. de Oliveira Filho, Anges Dossou, Sebastian Brünink, Melchior A. Joël Aïssi, Mamoudou Harouna Djingarey, Benjamin Hounkpatin, Michael Nagel
2, and Jan Felix Drexler
2
Author affiliations: Université Nationale des Sciences, Technologies, Ingénierie et Mathématiques, Cotonou, Benin (A. Yadouleton); Laboratoire des Fièvres Hémorragiques Virales du Benin, Cotonou (A. Yadouleton, C. Tchibozo, G. Hounkanrin, Y. Badou); Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany (A.-L. Sander, A. Moreira-Soto, C. Fischer, N. Krause, E.F. de Oliveira Filho, S. Brünink, J.F. Drexler); Ministry of Health, Cotonou (P. Akogbeto, A. Dossou, B. Hounkpatin); Conseil National de Lutte contre le VIH-Sida, la Tuberculose, le Paludisme, les IST et les Epidémies, Cotonou (M.A. Joël Aïssi); World Health Organization Regional Office for Africa, Health Emergencies Programme, Brazzaville, Congo (M.H. Djingarey); Deutsche Gesellschaft für Internationale Zusammenarbeit, Bonn, Germany (M. Nagel); German Centre for Infection Research, associated partner Charité-Universitätsmedizin, Berlin, Germany (J.F. Drexler)
Main Article
Figure 2
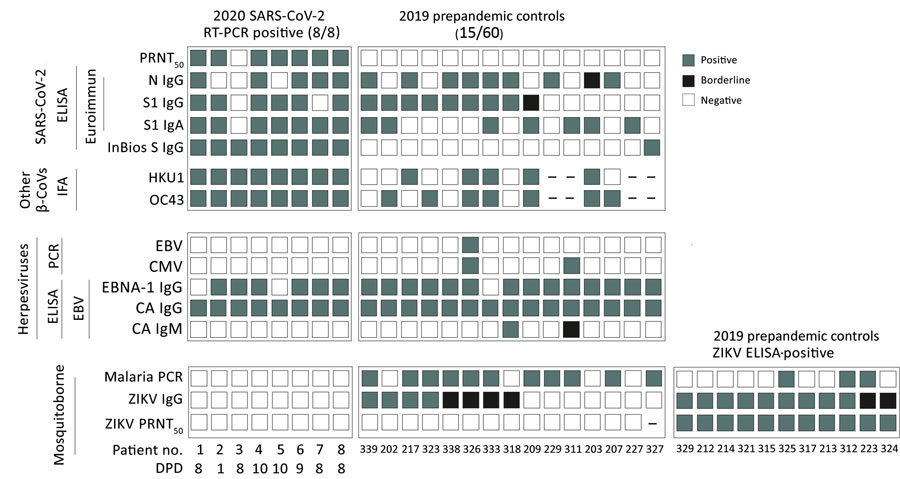
Figure 2. Molecular and serologic test results for betacoronaviruses and co-existing pathogens in Benin. Individual results are shown for reactivity of different commercially available SARS-CoV-2 ELISAs, SARS-CoV-2 PRNT, and IFA reactivity to common cold human coronaviruses OC43 and HKU1 in prepandemic controls from 2019 and SARS-CoV-2 RT-PCR confirmed patients from 2020; EBV PCR, CMV PCR, and 3 EBV ELISAs (EBV-CA IgM, EBV-CA IgG, and EBV-EBNA IgG) from the same groups; and ZIKV-IgG ELISA, ZIKV-PRNT, and malaria PCR from the same groups. Gray squares denote positive results; black squares denote inconclusive results; and white squares denote negative results. Dash (–) denotes samples in which the assay was not performed due to low sample volume. β-CoVs, betacoronaviruses; CA, viral capsid; CMV, cytomegalovirus; DPD, days the serum sample was taken after positive RT-PCR SARS-CoV-2 diagnosis; EBNA, nuclear antigen 1; EBV, Epstein-Barr virus; IFA, immunofluorescence; PRNT50, 50% plaque reduction neutralization test; RT-PCR, reverse transcription PCR; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ZIKV, Zika virus.
Main Article
Page created: November 02, 2020
Page updated: December 21, 2020
Page reviewed: December 21, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
