Volume 27, Number 10—October 2021
Dispatch
Therapeutic Efficacy of Human Monoclonal Antibodies against Andes Virus Infection in Syrian Hamsters
Figure 2
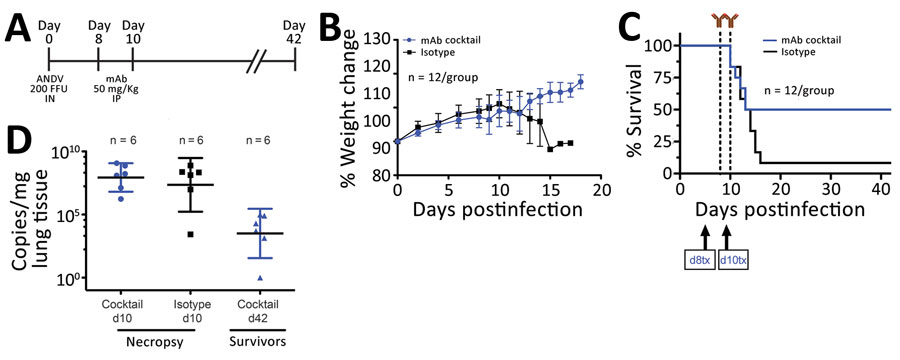
Figure 2. In vivo efficacy of a cocktail of human mAbs specific for ANDV glycoprotein administered at days 8 and 10 postinfection in the Syrian hamster model of hantavirus cardiopulmonary syndrome. A) Syrian hamsters were inoculated intranasally with 200 FFU of ANDV and then administered intraperitoneally a cocktail of mAbs (JL16 + MIB22; 25 mg/kg each) or isotype control (50 mg/kg) on day 8 and day 10 postinfection. B) Percentage of weight change monitored until 18 days postinfection, represented as the average per group. Error bars indicate 95% CIs. C) Statistical evaluation of survival by group. Survival was evaluated at p = 0.123 by Mantel-Cox log-rank test using GraphPad Prism (GraphPad Software, Inc., https://www.graphpad.com); p<0.05 was significant. d8 tx and d10 tx indicate treatment schedule (8 and 10 days postinfection). D) ANDV RNA copies per milligram of lung tissue on day 10 (d10) and day 42 (d42) postinfection. Samples were compared to a standard curve using an in vitro transcribed ANDV RNA fragment of known small segment copy number. Symbols indicate geometric means; horizontal line indicates median; error bars indicate 95% CIs. ANDV, Andes virus; FFU, focus-forming units; mAbs, monoclonal antibodies.
1These first authors contributed equally to this article.