Volume 27, Number 12—December 2021
Research
Novel Assay to Measure Seroprevalence of Zika Virus in the Philippines
Figure 2
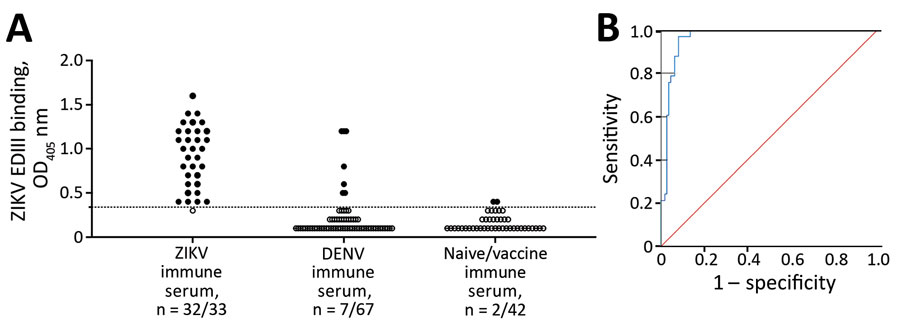
Figure 2. Performance evaluation of ZIKV EDIII assay in study of novel assay to measure ZIKV seroprevalence in the Philippines. Shown are the binding (A) and ROC (B) curve analysis of ZIKV EDIII ELISA using human convalescent serum samples. A panel of convalescent serum samples collected >12 weeks after onset of symptoms from primary and secondary ZIKV infections (n = 33), primary and multitypic DENV infections (n = 67), and serum samples collected >12 weeks after vaccination with a licensed Flavivirus vaccine or serum samples from Flavivirus-naive participants (n = 42) were tested by ZIKV EDIII ELISA. ROC demonstrated 0.966 (95% CI 0.94–0.99) area under the curve. The sensitivity of the EDIII capture ELISA was 97% (32/33) and the specificity 92% (100/109) at a cutoff value of 0.34. Red line indicates a random classifier and represents data points with equal true-positive rate and false-positive rate. The blue line is the ROC curve showing high performance of the ZIKV EDIII assay because the blue line is above and further away from a random classifier. DENV, dengue virus; EDIII, E protein domain III; OD405, optical density at 405 nm; ROC, receiver operating characteristic; ZIKV, Zika virus.
1Deceased.