Active Case Finding of Current Bornavirus Infections in Human Encephalitis Cases of Unknown Etiology, Germany, 2018–2020
Philip Eisermann
1, Dennis Rubbenstroth
1, Daniel Cadar, Corinna Thomé-Bolduan, Petra Eggert, Alexander Schlaphof, Frank Leypoldt, Martin Stangel, Thorsten Fortwängler, Florian Hoffmann, Andreas Osterman, Sabine Zange, Hans-Helmut Niller, Klemens Angstwurm, Kirsten Pörtner, Christina Frank, Hendrik Wilking, Martin Beer, Jonas Schmidt-Chanasit, and Dennis Tappe
Author affiliations: Bernhard-Nocht-Institut für Tropenmedizin, Hamburg, Germany (P. Eisermann, D. Cadar, C. Thomé-Bolduan, P. Eggert, A. Schlaphof, J. Schmidt-Chanasit, D. Tappe); Federal Research Institute for Animal Health, Greifswald-Insel Riems, Germany (D. Rubbenstroth, M. Beer); University Medical Center Schleswig-Holstein, Kiel, Germany (F. Leypoldt); Medizinische Hochschule Hannover, Hannover, Germany (M. Stangel); Donau-Isar-Klinikum Deggendorf, Deggendorf, Germany (T. Fortwängler); Ludwig-Maximilians-University, Munich, Germany (F. Hoffmann); Max von Pettenkofer Institute, Munich (A. Osterman); German Center for Infection Research, Munich (A. Osterman); Bundeswehr Institute of Microbiology, Munich (S. Zange); Regensburg University Hospital, Regensburg, Germany (H.-H. Niller, K. Angstwurm); Postgraduate Training for Applied Epidemiology, Berlin, Germany (K. Pörtner); European Centre for Disease Prevention and Control, Stockholm, Sweden (K. Pörtner); Robert Koch Institute, Berlin (K. Pörtner, C. Frank, H. Wilking)
Main Article
Figure 1
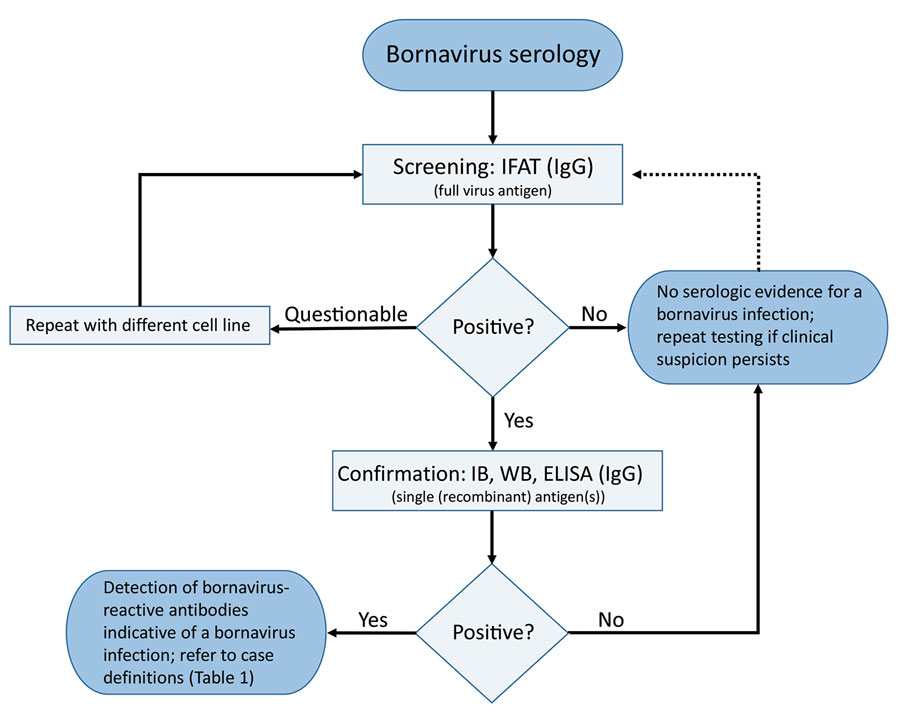
Figure 1. Serologic testing scheme for human bornavirus encephalitis, Germany, 2018–2020. Scheme was based on serologic screening and confirmatory assays and in conjunction with a case definition for variegated squirrel bornavirus 1 (VSBV-1) and Borna disease virus 1 (BoDV-1) encephalitis ([[ANCHOR###T1###Table 1###Anchor]]) was diagnosed. Screening of serum samples and cerebrospinal fluid for bornavirus-reactive IgG was conducted by using an indirect immunofluorescence antibody test. A persistently BoDV-1–infected cell line was used with uninfected cells of the same cell line as controls (Vero cells or Crandell-Rees feline kidney cells). For confirmation of a positive IFAT screening result, a line blot with recombinant VSBV-1 and BoDV-1 phosphoprotein proteins was used in our study, but alternative assays, such as WB or ELISA with recombinant antigen(s) or antigen(s) derived from infected cells, might also be appropriate after sufficient validation. Adequate control serum samples from confirmed human VSBV-1 and BoDV-1 encephalitis cases and a pooled serum of 20 healthy blood donors were used for the IFAT and the line blot. IFAT, indirect immunofluorescence antibody test; IB, immunoblot; WB, Western blotting.
Main Article
Page created: February 08, 2021
Page updated: April 20, 2021
Page reviewed: April 20, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
