Volume 27, Number 6—June 2021
Research
Twenty-Year Public Health Impact of 7- and 13-Valent Pneumococcal Conjugate Vaccines in US Children
Figure 2
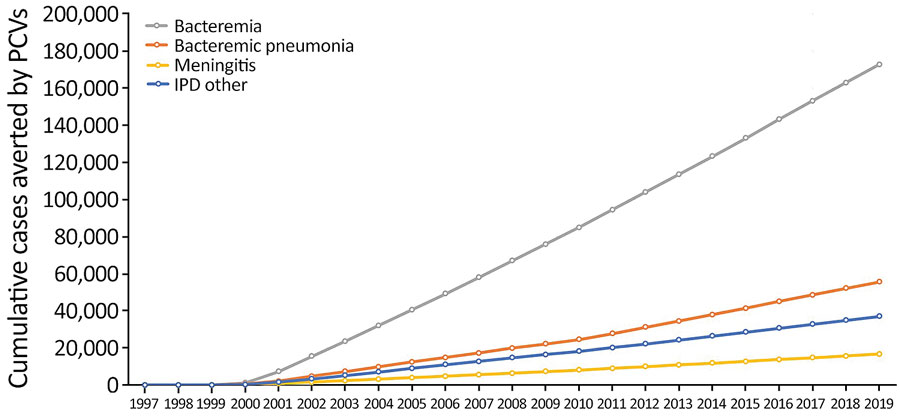
Figure 2. Effects of PCVs on different syndromes of IPD in children <5 years of age, United States, 1997–2019. The United States approved 7-valent PCV in 2000 and 13-valent PCV in 2010. IPD, invasive pneumococcal disease; PCV, pneumococcal conjugate vaccine.
Page created: April 15, 2021
Page updated: May 18, 2021
Page reviewed: May 18, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.