Volume 27, Number 7—July 2021
Research Letter
Effects of COVID-19 Vaccination Timing and Risk Prioritization on Mortality Rates, United States
Figure
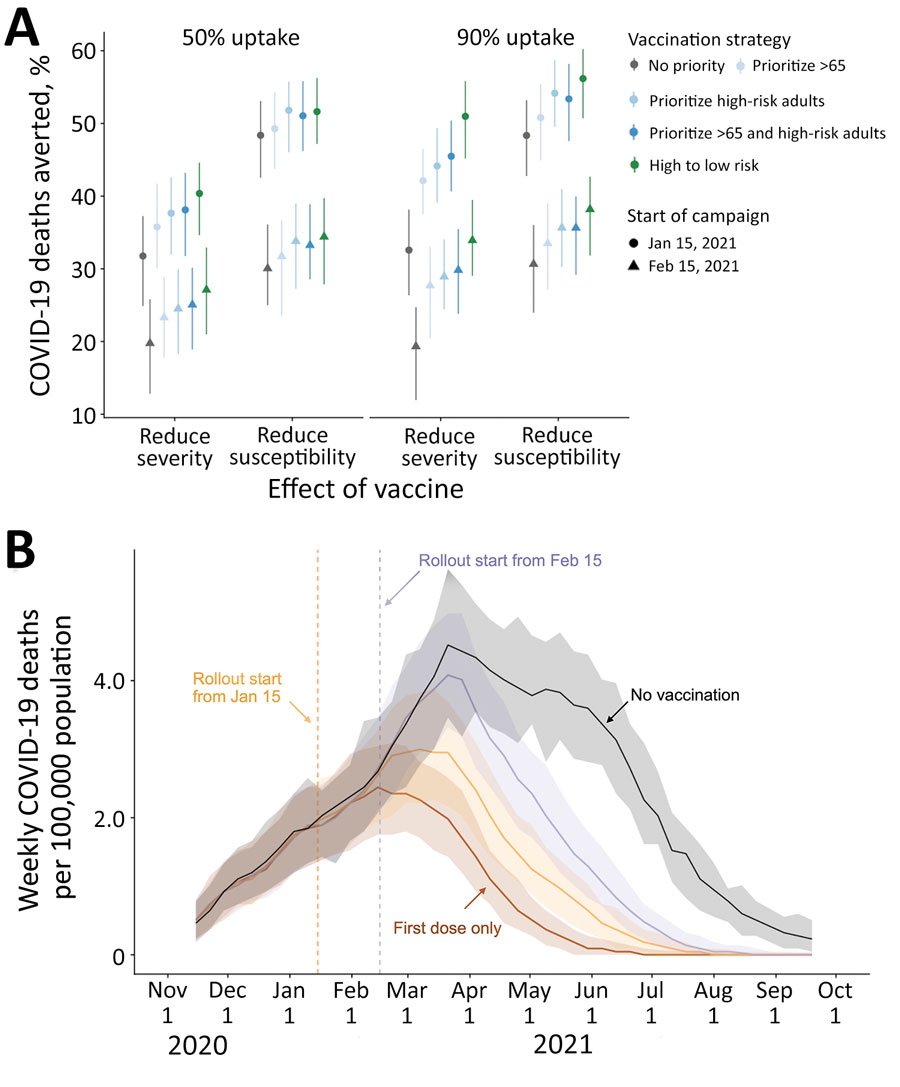
Figure. Projected COVID-19 deaths and deaths averted in the Austin–Round Rock Metropolitan Statistical Area (Austin, TX, USA) under various vaccine rollout scenarios for November 8, 2020–September 17, 2021. A) COVID-19 deaths averted after January 15, 2021, under combinations of vaccine uptake of 50% (left) or 90% (right); type of protection, either infection blocking (reducing susceptibility) or symptom blocking (reducing severity); rollout dates, either January 15 (circles) or February 15 (triangles); and risk prioritization, either no priority (gray), prioritize all adults >65 years of age (light blue), adults with high-risk underlying conditions (medium blue), or the combination of the two (dark blue), or a 10-phase risk-ordered strategy (green) that sequentially vaccinates >65 y high risk, 50–64 y high risk, >65 y low risk, 18–49 y high risk, 50–64 y low risk, 18–49 y low risk, 0–4 y high risk, 5–17 y high risk, 0–4 y low risk, 5–17 y low risk. Points and whiskers indicate the median and 95% CI across 200 paired stochastic simulations. B) Weekly incident COVID-19 deaths per 100,000 population, assuming intermediate (70%) uptake (6) without vaccine (black) or under a 10-phase risk-based rollout of a 95% efficacious infection-blocking vaccine, starting either January 15 (orange) or February 15 (purple). The brown line assumes that only first doses are administered starting January 15. Solid lines and shading indicate the median and 95% CI across 200 stochastic simulations. COVID-19, coronavirus disease.
References
- Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Moderna COVID-19 vaccine. December 17, 2020 [cited 2021 May 7]. https://www.fda.gov/media/144434/download
- Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Pfizer BioNTech COVID-19 vaccine. December 10, 2020 [cited 2021 May 7]. https://www.fda.gov/media/144245/download
- US Food and Drug Administration. COVID-19 vaccines. [cited 2021 Feb 17]. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines
- Dooling K, McClung N, Chamberland M, Marin M, Wallace M, Bell BP, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857–9. DOIPubMedGoogle Scholar
- US Centers for Disease Control and Prevention. Vaccines for COVID-19. 2021 [cited 2021 Feb 15]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Tyson A, Johnson C, Funk C. US public now divided over whether to get COVID-19 vaccine. 2020 [cited 2020 Dec 14]. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
- Pancevski B. UK delays second COVID-19 vaccine dose as Europe ponders how to speed up immunization. 2020 [cited 2021 Jan 13]. https://www.wsj.com/articles/u-k-delays-second-covid-19-vaccine-dose-as-europe-ponders-how-to-speed-up-immunization-11609334172
- US Food and Drug Administration. FDA statement on following the authorized dosing schedules for COVID-19 vaccines. January 4, 2021 [cited 2021 Jan 14]. https://www.fda.gov/news-events/press-announcements/fda-statement-following-authorized-dosing-schedules-covid-19-vaccines
- Livingston EH. Necessity of 2 doses of the Pfizer and Moderna COVID-19 vaccines. JAMA. 2021;325:898. DOIPubMedGoogle Scholar
- UK science advisers: publish evidence behind COVID vaccine changes. Nature. 2021;589:169–70. DOIPubMedGoogle Scholar