Volume 27, Number 8—August 2021
Dispatch
Weekly SARS-CoV-2 Sentinel Surveillance in Primary Schools, Kindergartens, and Nurseries, Germany, June‒November 2020
Abstract
We investigated severe acute respiratory syndrome coronavirus 2 infections in primary schools, kindergartens, and nurseries in Germany. Of 3,169 oropharyngeal swab specimens, only 2 were positive by real-time reverse transcription PCR. Asymptomatic children attending these institutions do not appear to be driving the pandemic when appropriate infection control measures are used.
Children have been disproportionately affected by public health measures in the current coronavirus disease (COVID-19) pandemic (1). In contrast to other age groups, children have shown lower rates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)‒positive cases; lower risk for symptomatic, acute, COVID-19; a generally milder course of disease with the exception of some rare manifestations and the post–COVID-19 multisystem inflammatory syndrome in children; and lower secondary attack rates (2–4). Susceptibility to infection in <10 years of age is estimated to be lower than that for teenagers. Accumulating evidence shows that, given limited infection control measures, SARS-CoV-2 might spread sustainably in secondary/high schools but to a lesser degree in primary schools and nurseries (2,5).
Closure of childcare facilities and schools has been shown to negatively affect the physical and emotional well-being of children, teenagers, and parents, potentially having a long-term impact on their lives (6). Thus, various expert groups called for avoiding closing of these institutions (7,8). Against the background of presymptomatic transmission found in adults, it is critical to public health authorities to be able to rely on real-life data monitoring the number of asymptomatic yet infected children attending educational institutions (9). Some studies have reported low numbers of infected cases in primary schools or childcare facilities but were conducted during a lockdown or semi-lockdown period (5,10). The aim of our study (the Münchner Virenwächter Study) was to implement a real-time sentinel program in a representative number of 5 primary schools and 5 (6 in phase 2) nurseries/kindergartens in Munich, Germany.
This study was approved by the ethics committee of the Ludwig-Maximilians University under project no. 20-484. We intended to accomplish a timely detection of infected cases and offer an additional level of safety to participating institutions during regular operating mode. The study spanned over 2 phases (Figure 1): phase 1, June 15‒July 26, 2020; and phase 2, September 7‒November 1, 2020. Participating institutions were randomly selected, and written informed consent was obtained in the first week of each phase. To correct for underrepresentation of younger children (<5 years of age), we included an additional nursery/kindergarten into phase 2.
We tested oropharyngeal swab specimens for SARS-CoV-2 by using real-time reverse transcription PCR (rRT-PCR); weekly samples were obtained from randomly selected children (n = 20) and staff (n = 5) in each institution. Swab specimens were taken on-site by trained medical personnel, and results were timely reported. For rRT-PCR, we processed specimens by using the AmpliCube Coronavirus SARS-CoV-2 Panel (Mikrogen, https://www.mikrogen.de) on a CFX96 Touch rRT-PCR Detection System (Bio-Rad, https://www.bio-rad.com). We retested single gene results by using the Xpert Xpress SARS-CoV-2 Test (Cepheid, https://www.cepheid.com).
We performed SARS-CoV-2 IgG screening at 3 sequential time points for samples from consenting staff members by using the Liaison SARS-CoV-2 S1/S2 IgG System (DiaSorin, https://www.diasorin.com) (Figure 1). We confirmed active results by using the RecomLine SARS-CoV-2 IgG Lineblot (Mikrogen). Antibody screening was complemented by obtaining a throat swab specimen at the same time to exclude active infection. Institutions were asked to respond to a questionnaire assessing implementation of infection control measures for phases 1 and 2.
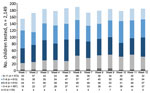
We processed 3,169 oropharyngeal swab specimens during the 12-week testing period, 2,149 from children (median age 7 years, range 1‒11 years, male:female ratio 1.03) and 1,020 from staff (median age 41 years, range 17–76 years, male:female ratio 0.13). We also obtained 493 swab specimens from staff during weekly testing and 527 swab specimens to complement serologic testing. We also tested 527 blood samples from staff for SARS-CoV-2 IgG. We determined pediatric sample distribution per study week (Figure 2).
No SARS-CoV-2 infections were detected during phase 1 of the study. During phase 2, only week 12 yielded 2 positive samples from 1 primary school. All SARS-CoV-2 IgG test results were negative at timepoints 1 and 2; only 1 positive serologic result was detected at timepoint 3. We identified some changes for implemented infection control measures between study phases and for individual facets between schools and childcare facilities (Table). All children attending primary schools were wearing face masks on school premises, except when seated for classes. Regular ventilation was begun as a daily routine in all institutions, as per national infection prevention and control guidance (Robert Koch Institute, https://www.rki.de).
Designed during the first lockdown in Munich, our study was intended to determine a feasible SARS-CoV-2 sentinel program in primary schools and childcare facilities in anticipation of a second pandemic wave and increasing incidence rates. Although public health and political authorities were concerned that childcare institutions would be major drivers of the pandemic, our results suggest that this did not happen. This result was consistent with those of another report suggesting that it was unlikely that children are major drivers of the pandemic even if attending schools (11).
Our study was not powered to accurately illustrate changes in incidences during low-incidence periods because of small sample sizes. However, we detected 2 cases in a primary school, 1 child and 1 teacher, during a high, local, 7-day incidence rate of 50 cases/100,000 children 1–11 years of age and 150 cases/100,000 persons in the general population. Tracing of 36 close contacts (33 classmates and 3 private contacts) identified only 1 additional case in another asymptomatic child in the same class. Telephone interview‒based contact tracing showed that the teacher reported to have experienced unspecific symptoms of headache and malaise 6 days before testing. Thus, it seems reasonable to deduce that transmission occurred from staff to both children.
Several reports have assessed the role of children in the dynamics of SARS-CoV-2 transmission. A study conducted in day care centers in Germany that used buccal mucosal and anal swabs for SARS-CoV-2 detection concluded that day care centers are not relevant reservoirs in a low prevalence setting (12). However, this study used self-testing, lacked oropharyngeal swab specimens, and was conducted during a minimal local incidence rate. Our study covered both low and high 7-day incidence periods while obtaining oropharyngeal swab specimens from children 1‒11 years of age. Ismail et al. reported complementary data from the United Kingdom, which showed that staff members had an increased risk for SARS-CoV-2 infection compared with students in any educational setting and that most cases linked to outbreaks were in staff (13).
Secondary attack rate analysis of the cases in our study also suggests that infections were transmitted from staff to children. In addition, low prevalence for SARS-CoV-2 antibodies in staff over the 3-month study period suggests no relevant infection activity in neither work nor private setting. Another recent report highlighted the need for maintaining low infection rates in the community to keep schools open during the pandemic (14).
Our study was conducted before the emergence of SARS-CoV-2 variants, such as B.1.1.7. Thus, the effect of this variant on children could not be addressed in our study. However, recent data from the United Kingdom found no evidence of more severe disease in children during the second wave, suggesting that infection with the B.1.1.7 variant does not result in a greatly different clinical course than the original strain (15).
We conclude that asymptomatic children attending primary schools, kindergartens, and nurseries are not greatly contributing to pandemic distribution of SARS-CoV-2 while adhering to infection control measures described above, even during high local background incidence. Thus, these children are unlikely to initiate clusters or outbreaks in the community when these institutions continue to play their critical role for the physical and emotional well-being of children and their families.
Dr. Hoch is head of the department of Infectious Diseases Epidemiology and Task Force Infectiology at the Bavarian Health and Food Safety Authority, Oberschleissheim, Germany. His primary research interests are pediatric infectious diseases and their impact on public health, and emerging and reemerging infectious diseases.
Acknowledgments
We thank Annalena Branz, Felix Flachenecker, Janina Ludwig, Adrian Rödig, Maria-Sophia Stadler, Jasmin Mahdawi, and Johannes Nowak for assistance with field work; Rüdiger von Kries for statistical advice; and all participating institutions, their staff, as well as all children and their parents, for valuable support.
This study was supported by the Bayerisches Staatsministerium für Unterricht und Kultus, the Referat für Bildung und Sport der Landeshauptstadt München, and the Referat für Gesundheit und Umwelt.
References
- Snape MD, Viner RM. COVID-19 in children and young people. Science. 2020;370:286–8. DOIPubMedGoogle Scholar
- Goldstein E, Lipsitch M, Cevik M. On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. J Infect Dis. 2021;223:362–9. DOIPubMedGoogle Scholar
- Kim-Hellmuth S, Hermann M, Eilenberger J, Ley-Zaporozhan J, Fischer M, Hauck F, et al. SARS-CoV-2 triggering severe acute respiratory distress syndrome and secondary hemophagocytic lymphohistiocytosis in a 3-year-old child with Down syndrome. J Pediatric Infect Dis Soc. 2021;10:543–6. DOIPubMedGoogle Scholar
- Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al.; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–69. DOIPubMedGoogle Scholar
- Ladhani SN, Baawuah F, Beckmann J, Okike IO, Ahmad S, Garstang J, et al. SARS-CoV-2 infection and transmission in primary schools in England in June-December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health. 2021;5:417–27. DOIPubMedGoogle Scholar
- Buonsenso D, Roland D, De Rose C, Vásquez-Hoyos P, Ramly B, Chakakala-Chaziya JN, et al. Schools closures during the COVID-19 pandemic: a catastrophic global situation. Pediatr Infect Dis J. 2021;40:e146–50. DOIPubMedGoogle Scholar
- Levinson M, Cevik M, Lipsitch M. Reopening primary schools during the pandemic. N Engl J Med. 2020;383:981–5. DOIPubMedGoogle Scholar
- Patrick SW, Henkhaus LE, Zickafoose JS, Lovell K, Halvorson A, Loch S, et al. Well-being of parents and children during the COVID-19 pandemic: a national survey. Pediatrics. 2020;146:
e2020016824 . DOIPubMedGoogle Scholar - L’Huillier AG, Torriani G, Pigny F, Kaiser L, Eckerle I. Culture-competent SARS-CoV-2 in nasopharynx of symptomatic neonates, children, and adolescents. Emerg Infect Dis. 2020;26:2494–7. DOIPubMedGoogle Scholar
- Otte Im Kampe E, Lehfeld A-S, Buda S, Buchholz U, Haas W. Surveillance of COVID-19 school outbreaks, Germany, March to August 2020. Euro Surveill. 2020;25:25. DOIPubMedGoogle Scholar
- Soriano-Arandes A, Gatell A, Serrano P, Biosca M, Campillo F, Capdevila R et al.; the COPEDI-CAT Research Group. Household SARS-CoV-2 transmission and children: a network prospective study. Clib Dis. 20210;Mar 12:ciaa228 [Online ahead of print].
- Hoehl S, Kreutzer E, Schenk B, Westhaus S, Foppa I, Herrmann E et al. Longitudinal testing for respiratory and gastrointestinal shedding of SARS-CoV-2 in day care centres in Hesse, Germany. Clin Infect Dis. 2021;Jan 3:ciaa1912 [Online ahead of print].
- Ismail SA, Saliba V, Lopez Bernal J, Ramsay ME, Ladhani SN. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021;21:344–53. DOIPubMedGoogle Scholar
- Mensah AA, Sinnathamby M, Zaidi A, Coughlan L, Simmons R, Ismail SA, et al. SARS-CoV-2 infections in children following the full re-opening of schools and the impact of national lockdown: Prospective, national observational cohort surveillance, July-December 2020, England. J Infect. 2021;82:67–74. DOIPubMedGoogle Scholar
- Brookman S, Cook J, Zucherman M, Broughton S, Harman K, Gupta A. Effect of the new SARS-CoV-2 variant B.1.1.7 on children and young people. Lancet Child Adolesc Health. 2021;5:e9–10. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: June 03, 2021
1These authors contributed equally to this article.
Table of Contents – Volume 27, Number 8—August 2021
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ulrich von Both, Hauner Children’s Hospital, Ludwig-Maximilians-University, Lindwurmstrasse 4, 80337 Munich, Germany
Top