Volume 28, Number 10—October 2022
Research Letter
Emerging Tickborne Bacteria in Cattle from Colombia
Abstract
Ehrlichia minasensis is a new pathogenic bacterial species that infects cattle, and Borrelia theileri causes bovine borreliosis. We detected E. minasensis and B. theileri DNA in cattle from southwestern Colombia by using PCR. E. minasensis and B. theileri should be considered potential etiologies of febrile syndrome in cattle from Colombia.
Ehrlichia spp. are tickborne obligate intracellular bacteria and comprise different pathogenic species that affect both veterinary and public health (1). Ehrlichia minasensis was first detected in cattle and deer in Canada and later in cattle and Rhipicephalus microplus ticks from Brazil (2–4). Infected cattle manifest signs that include fever, lethargy, depression, and anorexia (3,4). Borrelia theileri belongs to the relapsing fever group of borreliae and causes bovine borreliosis, which is a mild febrile disease associated with lethargy, hemoglobinuria, and anemia (5). This spirochete is transmitted by Rhipicephalus (formerly Boophilus) sp. ticks and has been documented in Africa, Europe, Oceania, and South America (5,6). To our knowledge, E. minasensis or B. theileri infections have not been reported in cattle from Colombia.
During September and October 2017, we collected blood samples from 30 bovids with tick parasitism in El Tambo and Santander de Quilichao municipalities, Cauca department, Colombia (Appendix Figure). We extracted DNA from blood using the QIAGEN DNeasy Blood & Tissue Kit (https://www.qiagen.com), according to the manufacturer’s instructions. We verified DNA quality using PCR amplification of the vertebrate cytochrome B gene CYTB. We subsequently performed PCR to detect dsb and trp36 genes for Ehrlichia spp.; flaB and 16S rRNA genes for Borrelia spp.; and rpoB, msp4, and msp1a genes for Anaplasma spp. (Appendix Table). DNA samples that produced strong PCR bands underwent sequencing on an Applied Biosystems 3130/3130xl Genetic Analyzer (Thermo Fisher Scientific, https://www.thermofisher.com). The msp1a PCR products were poor quality and did not undergo sequencing. We aligned sequences using GeneStudio (GeneStudio, Inc., https://genestudio-pro.software.informer.com) and performed multiple sequence alignments using the EMBL-European Bioinformatics Institute tools MUSCLE (for Ehrlichia spp.) and ClustalW (for Borrelia and Anaplasma spp.) (https://www.clustal.org). Phylogenetic analyses were performed with MEGA X software (https://www.megasoftware.net). We generated phylogenetic trees for dsb, flaB, and 16S rRNA genes and Trp36 protein using the maximum-likelihood estimation method. All procedures were approved by the Pontificia Universidad Javeriana Ethics Committee in Colombia.
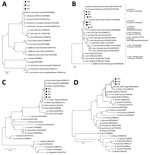
We detected CYTB in all samples. We detected the dsb gene in 10% (3/30) and trp36 in 20% (6/30) of samples. The flaB gene was detected in 13.3% (4/30), and the 16S rRNA gene was detected in 10% (3/30) of samples. The rpoB gene was amplified in 90% (27/30), msp4 was amplified in 83.3% (25/30), and msp1a was amplified in 83.3% (25/30) of samples. We performed phylogenetic analyses of 3 sequences for dsb (GenBank accession nos. ON209405–7), 6 sequences for Trp36 protein (inferred from GenBank accession nos. OL513405–10), 3 sequences for the 16S rRNA gene (GenBank accession nos. ON112216–8), 4 sequences for flaB (GenBank accession nos. ON135431–4); 6 sequences for rpoB (GenBank accession nos. ON209412–7), and 4 sequences for msp4 (GenBank accession nos. ON209408–11).
Phylogenetic analyses showed that our dsb gene sequences clustered with E. minasensis dsb sequences from Brazil, Australia, and Colombia (Figure, panel A). The greater genetic diversity of Trp36 protein compared with dsb provided more detailed characterization of Ehrlichia sp. genotypes. Our Trp36 sequences clustered with 3 sequences from Brazil and a recently described E. minasensis strain from China isolated from Haemaphysalis hystricis ticks (Figure, panel B). The flaB and 16S rRNA genes clustered with B. theileri sequences (Figure, panels C, D). Bootstrap values were 86% for flaB and 72% for the 16S rRNA gene. The flaB sequences grouped with sequences from Argentina, Republic of the Congo, Egypt, and Israel (Figure, panel C). The 16S rRNA gene sequences grouped independently but close to sequences from Egypt and the United States (Figure, panel D). We confirmed A. marginale using identity analysis (100% identical to GenBank sequences; accession nos. CP023731, CP006846, CP001079, CP000030, and AF428086) of rpoB and msp4 genes. Co-infection with E. minasensis and A. marginale was confirmed in 6 animals from El Tambo. Co-infection with B. theileri and A. marginale was documented in 1 animal from El Tambo and 1 animal from Santander de Quilichao.
These results showed the simultaneous circulation of E. minasensis, B. theileri, and A. marginale in bovids from Cauca department, Colombia. In Latin America, E. minasensis has been identified in Brazil (7) and Colombia (found in R. microplus ticks) (8), and B. theileri has been found in Argentina (6), Mexico (9), and Brazil (10). The R. microplus tick is likely the main vector for both pathogens in these regions and has been confirmed by molecular detection of E. minasensis in tick specimens collected from the same animals (H.C. Martínez-Diaz, unpub. data) and various reports in Latin America for B. theileri (9,10). Despite the lack of clinical signs in these animals, tickborne infections caused by these pathogenic bacteria often occur as subclinical infections or with intermittent clinical manifestations. E. minasensis and B. theileri infections, either separately or as co-infections, may be more frequent than previously recognized and should be considered potential etiologies of febrile syndrome in cattle from this and other regions of Colombia.
Dr. Ramírez-Hernández is a researcher at Universidad de La Salle, Bogotá D.C., Colombia. His research interests focus on ticks, fleas, and ecoepidemiology of tickborne and fleaborne diseases.
Acknowledgments
We thank all personnel involved in sample collection in Cauca, Colombia.
This research was supported by COLCIENCIAS, Colombia (code no. 120374455209) and the Fogarty International Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (award no. 5D43TW010331-05). Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Ismail N, McBride JW. Tick-borne emerging infections: ehrlichiosis and anaplasmosis. Clin Lab Med. 2017;37:317–40. DOIPubMedGoogle Scholar
- Cabezas-Cruz A, Zweygarth E, Vancová M, Broniszewska M, Grubhoffer L, Passos LMF, et al. Ehrlichia minasensis sp. nov., isolated from the tick Rhipicephalus microplus. Int J Syst Evol Microbiol. 2016;66:1426–30. DOIPubMedGoogle Scholar
- Moura de Aguiar D, Pessoa Araújo Junior J, Nakazato L, Bard E, Aguilar-Bultet L, Vorimore F, et al. Isolation and characterization of a novel pathogenic strain of Ehrlichia minasensis. Microorganisms. 2019;7:528. DOIPubMedGoogle Scholar
- Aguiar DM, Ziliani TF, Zhang X, Melo AL, Braga IA, Witter R, et al. A novel Ehrlichia genotype strain distinguished by the TRP36 gene naturally infects cattle in Brazil and causes clinical manifestations associated with ehrlichiosis. Ticks Tick Borne Dis. 2014;5:537–44. DOIPubMedGoogle Scholar
- Elelu N. Tick-borne relapsing fever as a potential veterinary medical problem. Vet Med Sci. 2018;4:271–9. DOIPubMedGoogle Scholar
- Morel N, De Salvo MN, Cicuttin G, Rossner V, Thompson CS, Mangold AJ, et al. The presence of Borrelia theileri in Argentina. Vet Parasitol Reg Stud Rep. 2019;17:
100314 . DOIPubMedGoogle Scholar - Cabezas-Cruz A, Zweygarth E, Aguiar DM. Ehrlichia minasensis, an old demon with a new name. Ticks Tick Borne Dis. 2019;10:828–9. DOIPubMedGoogle Scholar
- Miranda J, Mattar S. Molecular detection of Anaplasma sp. and Ehrlichia sp. in ticks collected in domestical animals, Colombia. Trop Biomed. 2015;32:726–35.PubMedGoogle Scholar
- Smith RD, Brener J, Osorno M, Ristic M. Pathobiology of Borrelia theileri in the tropical cattle tick, Boophilus microplus. J Invertebr Pathol. 1978;32:182–90. DOIPubMedGoogle Scholar
- Martins JR, Ceresér VH, Corrêa BL, Smith RD. Borrelia theileri: observação em carrapatos do gênero Boophilus microplus no município de Guaíba, RS, Brasil. Ciência Rural, Santa Maria. 1996;26:447–50. DOIGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: September 09, 2022
Table of Contents – Volume 28, Number 10—October 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
David H. Walker, Department of Pathology, University of Texas Medical Branch, 301 University Blvd, Galveston, TX, 77555-0609, USA
Top