Volume 28, Number 11—November 2022
Dispatch
Seroincidence of Enteric Fever, Juba, South Sudan
Figure 1
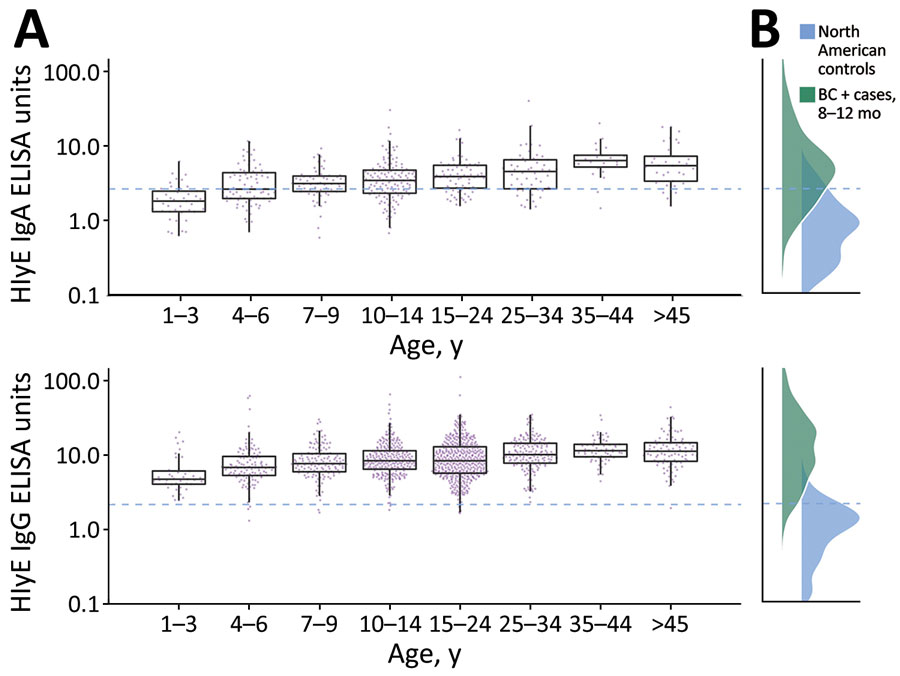
Figure 1. Age-dependent hemolysin E (HlyE) IgA (top) and IgG (bottom) responses for participants in study of seroincidence of enteric fever, Juba, South Sudan, 2020, compared with those for blood culture-confirmed cases and controls. A) Cross-sectional antibody responses to HlyE IgA (top) and IgG (bottom) by age measured from a serosurvey of 1,290 persons in Juba, South Sudan, from samples during collected during August 7–September 2, 2020. Each point indicates an individual sample. Horizontal lines within boxes indicate medians; box tops and bottoms indicate IQRs; error bars indicate 95% CIs. B) Density of antibody responses HlyE IgA (top) and IgG (bottom) among 1,410 blood-culture confirmed enteric fever cases in Bangladesh, Nepal, Pakistan, and Ghana 8–12 months after symptom onset as reported in (12) and a control population from 3 United States groups: 48 children 1–5 years of age who had first degree relatives with celiac disease, enrolled nationally; 31 healthy controls, children and young adults 2–18 years of age, enrolled at Massachusetts General Hospital (Appendix reference 17); and a population-based sample of 205 children and adults 3–50 years of age participating in a SARS-CoV serosurvey in California, USA. The dashed blue line across all panels represents the mean +3 SD of HlyE IgA and IgG values observed in the pediatric control population. HlyE, hemolysin E.
References
- Stanaway JD, Reiner RC, Blacker BF, Goldberg EM, Khalil IA, Troeger CE, et al.; GBD 2017 Typhoid and Paratyphoid Collaborators. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19:369–81. DOIPubMedGoogle Scholar
- Antillon M, Saad NJ, Baker S, Pollard AJ, Pitzer VE. The relationship between blood sample volume and diagnostic sensitivity of blood culture for typhoid and paratyphoid fever: a systematic review and meta-analysis. J Infect Dis. 2018;218(suppl_4):S255–67. DOIPubMedGoogle Scholar
- Voysey M, Pant D, Shakya M, Liu X, Colin-Jones R, Theiss-Nyland K, et al. Under-detection of blood culture-positive enteric fever cases: The impact of missing data and methods for adjusting incidence estimates. PLoS Negl Trop Dis. 2020;14:
e0007805 . DOIPubMedGoogle Scholar - Azman AS, Bouhenia M, Iyer AS, Rumunu J, Laku RL, Wamala JF, et al. High hepatitis E seroprevalence among displaced persons in South Sudan. Am J Trop Med Hyg. 2017;96:1296–301. DOIPubMedGoogle Scholar
- Abubakar A, Bwire G, Azman AS, Bouhenia M, Deng LL, Wamala JF, et al. Cholera epidemic in South Sudan and Uganda and need for international collaboration in cholera control. Emerg Infect Dis. 2018;24:883–7. DOIPubMedGoogle Scholar
- Kumar S, Nodoushani A, Khanam F, DeCruz AT, Lambotte P, Scott R, et al. Evaluation of a rapid point-of-care multiplex immunochromatographic assay for the diagnosis of enteric fever. MSphere. 2020;5:e00253–20. DOIPubMedGoogle Scholar
- Andrews JR, Khanam F, Rahman N, Hossain M, Bogoch II, Vaidya K, et al. Plasma immunoglobulin A responses against 2 Salmonella Typhi antigens identify patients with typhoid fever. Clin Infect Dis. 2019;68:949–55. DOIPubMedGoogle Scholar
- McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet. 2004;36:1268–74. DOIPubMedGoogle Scholar
- Charles RC, Liang L, Khanam F, Sayeed MA, Hung C, Leung DT, et al. Immunoproteomic analysis of antibody in lymphocyte supernatant in patients with typhoid fever in Bangladesh. Clin Vaccine Immunol. 2014;21:280–5. DOIPubMedGoogle Scholar
- Charles RC, Sheikh A, Krastins B, Harris JB, Bhuiyan MS, LaRocque RC, et al. Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients by an immunoaffinity proteomics-based technology. Clin Vaccine Immunol. 2010;17:1188–95. DOIPubMedGoogle Scholar
- Charles RC, Sultana T, Alam MM, Yu Y, Wu-Freeman Y, Bufano MK, et al. Identification of immunogenic Salmonella enterica serotype Typhi antigens expressed in chronic biliary carriers of S. Typhi in Kathmandu, Nepal. PLoS Negl Trop Dis. 2013;7:
e2335 . DOIPubMedGoogle Scholar - Aiemjoy K, Seidman JC, Saha S, Munira SJ, Islam Sajib MS, Sium SMA, et al. Estimating typhoid incidence from community-based serosurveys: a multicohort study. Lancet Microbe. 2022;3:e578–87. DOIPubMedGoogle Scholar
- Wiens KE, Mawien PN, Rumunu J, Slater D, Jones FK, Moheed S, et al. Seroprevalence of severe acute respiratory syndrome coronavirus 2 IgG in Juba, South Sudan. Emerg Infect Dis. 2021;27:1598–606. DOIPubMedGoogle Scholar
- Garrett DO, Longley AT, Aiemjoy K, Yousafzai MT, Hemlock C, Yu AT, et al. Incidence of typhoid and paratyphoid fever in Bangladesh, Nepal, and Pakistan: results of the Surveillance for Enteric Fever in Asia Project. Lancet Glob Health. 2022;10:e978–88. DOIPubMedGoogle Scholar
- Marks F, von Kalckreuth V, Aaby P, Adu-Sarkodie Y, El Tayeb MA, Ali M, et al. Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health. 2017;5:e310–23. DOIPubMedGoogle Scholar