Volume 28, Number 12—December 2022
Research Letter
Hemotropic Mycoplasma spp. in Aquatic Mammals, Amazon Basin, Brazil
Abstract
Hemotropic Mycoplasma spp. (hemoplasmas) are uncultivable bacteria that infect mammals, including humans. We detected a potentially novel hemoplasma species in blood samples from wild river dolphins in the Amazon River Basin, Brazil. Further investigation could determine pathogenicity and zoonotic potential of the detected hemoplasma.
Hemotropic Mycoplasma spp. (hemoplasmas) are uncultivable, cell-wall–deficient, pleomorphic bacteria that infect mammals, including humans (1). Although previously linked to anemia, starvation, and death, especially among immunosuppressed humans and animals (2,3), most hemoplasma species have subclinical manifestations (1). Hemoplasmas are thought to be host specific, but some reports suggest interspecies transmission and zoonotic potential (3–5). In aquatic mammals, hemoplasmas have only been reported in California sea lions (Zalophus californianus) (6).
Amazon river dolphins (Inia geoffrensis), Bolivian river dolphins (I. boliviensis), and Amazonian manatees (Trichechus inunguis) are endemic to the Amazon Basin. Both dolphin species have been classified as endangered, and T. inunguis manatees are classified as vulnerable (7). Infectious disease studies in these species are scarce. We used 16S rRNA PCR to detect and characterize hemoplasmas among aquatic mammals from the Amazon Basin Region, Brazil.
We analyzed blood samples of 50 wild dolphins, including 32 I. geoffrensis and 18 I. boliviensis dolphins live captured in scientific expeditions (8), during 2015 in the Guaporé and Negro Rivers; 2017 in the Tapajós River; and 2020 near Balbina hydroelectric dam (Table). We performed field hematology on wild dolphins and also analyzed blood samples collected during health assessments of 25 T. inunguis manatees under human care in Manaus in February 2022 (Appendix Tables 1, 2).
We extracted DNA by using the DNeasy Blood & Tissue Kit (QIAGEN, https://www.qiagen.com), following manufacturer instructions. We screened samples for Mycoplasma spp. by 16S rRNA PCR targeting a 384-bp fragment (9). We subjected positive samples to PCR targeting a 1,400-bp fragment of 16S rRNA (10) and confirmed amplicons by sequencing in both directions.
We used GraphPad Prism version 5 (GraphPad Software, https://www.graphpad.com) to compare prevalence among host species, sampling sites, sampling year, age, and sex, and hematological values in infected and noninfected animals; we considered p<0.05 statistically significant. We used the median joining method in PopART software (University of Otagao, https://www.popart.otago.ac.nz) to generate a nucleotide sequence type network. We assessed phylogeographic structure among species and sampling sites by using pairwise fixation index tests (FSTs) in Arlequin (http://cmpg.unibe.ch/software/arlequin3), determining level of significance with 1,000 permutations, and using the nearest-neighbor statistic (Snn) in DnaSP version 5 (Universitat de Barcelona, http://www.ub.edu/dnasp).
We detected Mycoplasma DNA in samples from 21 (65.6%, 95% CI 48.2%–83.0%) I. geoffrensis and 11 (61.1%, 95% CI 36.2%–86.1%) I. boliviensis dolphins. The percentage of Inia spp. dolphins testing hemoplasma-positive was higher than that reported for Z. californianus California sea lions (12.4%) (6). All manatees in our study tested PCR-negative for hemoplasma.
Mycoplasma nucleotide sequences from Inia spp. dolphins had <94.0% identity with the closest available sequence (GenBank accession no. CP003731), which was detected in alpacas (Vicugna pacos). We submitted 12 representative sequence types to GenBank (Table). Multilocus sequencing typing will be necessary to further characterize the Mycoplasma species we detected.
Among animals sampled, adult dolphins had significantly higher hemoplasma prevalence than did calves (p = 0.0015). We saw no statistically significant differences among remaining variables, including the hematologic parameters between hemoplasma-positive and hemoplasma-negative dolphins; however, our sample size was small.
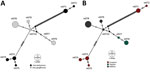
Network analyses differentiated the obtained nucleotide sequence types into 3 distinct groups: 1 comprises sequences of all I. geoffrensis dolphins samples from Balbina and Tapajós; the other 2, harbor sequences of all I. boliviensis dolphins samples from Guaporé, which are greatly divergent (Figure). Our analysis showed statistically significant differences among populations (Snn = 1.0, p = 0.0001; FST = 0.48, p = 0.003), confirming a geographic genetic structure. Haplotype diversity (Hd), average number of nucleotide differences (K), and nucleotide diversity (π) were higher among animals from Guaporé compared with the other 2 sites. For Guaporé, Hd was 0.82, K 43.6, and π 0.03; for Tapajós, Hd was 0.4, K 0.4, and π 0.0003; and for Balbina, Hd was 0.44, K 0.71, and π 0.0005. We also noted that Mycoplasma among host species shared genetic structure that differed between the 2 Inia species (Snn = 1.0, p = 0.0001; FST = 0.43, p = 0.000). The genetic structure difference between the species and sites likely reflects geographic separation of the studied populations (Appendix Figure 1). However, geographic separation does not explain the hemoplasma divergence between the 2 sequence types collected from I. boliviensis dolphins. All retrieved sequences clustered together and with other hemoplasma sequences of unknown pathogenicity (Appendix Figure 2).
Our findings indicate that aquatic mammals can be infected by hemoplasmas, but epidemiology remains unknown. In terrestrial mammals, hematophagous vectors are the main proposed transmission route (1). T. inunguis manatees in our study tested hemoplasma-negative despite being housed in tanks close to the forest without vector protection. This finding suggests food could be a transmission route among aquatic mammals because river dolphins are piscivorous and manatees are herbivorous. Also, 5 female dolphins captured with calves tested positive, but the calves tested negative, which might exclude vertical transmission. Endoparasitism or direct contact are other possible transmission routes.
In conclusion, we detected hemoplasmas in I. geoffrensis and I. boliviensis river dolphins. Pathogenicity and zoonotic potential require further investigation, but the high hemoplasma prevalence in adult mammals and detection among animals over several years suggest hemoplasma endemicity in these dolphin populations.
Dr. Duarte-Benvenuto is a veterinarian and a doctorate student at the Laboratory of Wildlife Comparative Pathology in University of São Paulo, Brazil. Her primary research interest is wildlife disease and conservation, especially of aquatic mammals.
Acknowledgments
This study was funded by Brazilian National Council for Scientific and Technological Development (scholarship no. 141868/2019-8 and fellowship no. 304999-18), Fundação de Amparo à Pesquisa do Estado de São Paulo (scholarship no. 2016/20956-0 and grant no. 2018/25069-7), and by the Juan the la Cierva incorporación and formación fellowship nos. IJC2020-046019-I and FJC2020-046311-1, the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Small Grant in Aid of Research from the Society for Marine Mammalogy.
The Amazon river dolphins from the Tapajós River were sampled as part of the South American River Dolphin (SARDI) integrated strategy for the conservation funded by the World Wildlife Fund. The Amazon river dolphins from the Negro River and Balbina hydroelectric dam were sampled as part of Projeto Mamíferos Aquáticos da Amazonia, sponsored by Ampa/Petrobras Socioambiental, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001and by the Fundação de Amparo à Pesquisa do Estado do Amazonas (grant/award no. UNIVERSAL AMAZONAS/062.00891/2019). All study samples were collected in full compliance with specific federal permits issued by the Brazil Ministry of Environment (MMA) and the Chico Mendes Institute for Biodiversity Conservation (ICMBio) and approved by the Biodiversity Information and Authorization System (SISBIO authorization nos. 31226-1/2, 47780-4, 49597-1, 60171-1,72608-1, and 76904-3.), and ABIO no. 1169/2019, ICMBio/MMA (authorization no. 13157), and SISGEN authorization no. AAF009C.
References
- Millán J, Di Cataldo S, Volokhov DV, Becker DJ. Worldwide occurrence of haemoplasmas in wildlife: Insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound Emerg Dis. 2021;68:3236–56. DOIPubMedGoogle Scholar
- Sykes JE, Tasker S. Hemoplasma infections. In: Sykes JE, editor. Canine and feline infectious diseases. St. Louis: Saunders; 2013. p. 390–398.
- Descloux E, Mediannikov O, Gourinat AC, Colot J, Chauvet M, Mermoud I, et al. Flying fox hemolytic fever, description of a new zoonosis caused by Candidatus Mycoplasma haemohominis. Clin Infect Dis. 2021;73:e1445–53. DOIPubMedGoogle Scholar
- dos Santos AP, dos Santos RP, Biondo AW, Dora JM, Goldani LZ, de Oliveira ST, et al. Hemoplasma infection in HIV-positive patient, Brazil. Emerg Infect Dis. 2008;14:1922–4. DOIPubMedGoogle Scholar
- Sacristán I, Acuña F, Aguilar E, García S, López MJ, Cevidanes A, et al. Assessing cross-species transmission of hemoplasmas at the wild-domestic felid interface in Chile using genetic and landscape variables analysis. Sci Rep. 2019;9:16816. DOIPubMedGoogle Scholar
- Volokhov DV, Norris T, Rios C, Davidson MK, Messick JB, Gulland FM, et al. Novel hemotrophic mycoplasma identified in naturally infected California sea lions (Zalophus californianus). Vet Microbiol. 2011;149:262–8. DOIPubMedGoogle Scholar
- International Union for Conservation of Nature and Natural Resources. The IUCN red list of threatened species [cited 2021 Jun 18]. https://www.iucnredlist.org
- da Silva VMF, Martin AR. A study of the Boto, or Amazon River Dolphin (Inia geoffrensis), in the Mamirauá Reserve, Brazil: operation and techniques. Occas Pap IUCN Species Surviv Comm. 2001;23:121–30.
- Cabello J, Altet L, Napolitano C, Sastre N, Hidalgo E, Dávila JA, et al. Survey of infectious agents in the endangered Darwin’s fox (Lycalopex fulvipes): high prevalence and diversity of hemotrophic mycoplasmas. Vet Microbiol. 2013;167:448–54. DOIPubMedGoogle Scholar
- Harasawa R, Orusa R, Giangaspero M. Molecular evidence for hemotropic Mycoplasma infection in a Japanese badger (Meles meles anakuma) and a raccoon dog (Nyctereutes procyonoides viverrinus). J Wildl Dis. 2014;50:412–5. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: November 15, 2022
Table of Contents – Volume 28, Number 12—December 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Aricia Duarte-Benvenuto, Laboratório de Patologia Experimental e Comparada, 87 Prof. Dr. Orlando Marques de Paiva Ave, São Paulo 05508270, Brazil; email address:aricia.benvenuto@gmail.com
Top