Novel Hendra Virus Variant Detected by Sentinel Surveillance of Horses in Australia
Edward J. Annand
1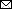
, Bethany A. Horsburgh
1, Kai Xu, Peter A. Reid, Ben Poole, Maximillian C. de Kantzow, Nicole Brown, Alison Tweedie, Michelle Michie, John D. Grewar, Anne E. Jackson, Nagendrakumar B. Singanallur, Karren M. Plain, Karan Kim, Mary Tachedjian, Brenda van der Heide, Sandra Crameri, David T. Williams, Cristy Secombe, Eric D. Laing, Spencer Sterling, Lianying Yan, Louise Jackson, Cheryl Jones, Raina K. Plowright, Alison J. Peel, Andrew C. Breed, Ibrahim Diallo, Navneet K. Dhand, Philip N. Britton, Christopher C. Broder, Ina Smith
2, and John-Sebastian Eden
2
Author affiliations: EquiEpiVet, Equine Veterinary and One Health Epidemiology, Aireys Inlet, Victoria, Australia (E.J. Annand); Department of Agriculture, Water, and the Environment Epidemiology and One Health Section, Canberra (E.J. Annand, M.C. de Kantzow, A.C. Breed); University of Sydney School of Veterinary Science and Institute for Infectious Diseases, Sydney, New South Wales, Australia (E.J. Annand, N. Brown, A. Tweedie, A.E. Jackson, K.M. Plain, N.K. Dhand); CSIRO Health and Biosecurity Black Mountain Laboratories, Canberra, Australian Capital Territory, Australia (E.J. Annand, M. Michie, I. Smith); Westmead Institute for Medical Research, Sydney (B.A. Horsburgh, K. Kim, J.-S. Eden); University of Sydney School of Medicine, Sydney (B.A. Horsburgh, C. Jones, P.N. Britton, J.-S. Eden); Ohio State University College of Veterinary Medicine, Columbus, Ohio, USA (K. Xu); Private equine veterinary practice, Brisbane, Queensland, Australia (P.A. Reid); Cooroora Veterinary Clinic, Cooroy, Queensland, Australia (B. Poole); JData, Cape Town, South Africa (J.D. Grewar); University of Pretoria, Pretoria, South Africa (J.D. Grewar); CSIRO Australian Centre for Disease Preparedness, Geelong, Victoria, Australia (N.B. Singanallur, M. Tachedjian, B. van der Heide, S. Crameri, D.T. Williams); Murdoch University School of Veterinary Medicine and The Animal Hospital, Murdoch, Western Australia, Australia (C. Secombe); Uniformed Services University of the Health Sciences Microbiology and Immunology, Bethesda, Maryland, USA (E.D. Laing, S. Sterling, L. Yan, C.C. Broder); Queensland Department of Agriculture and Fisheries Biosecurity Sciences Laboratory, Brisbane (L. Jackson, I. Diallo); Children’s Hospital at Westmead, Infectious Diseases, Sydney (C. Jones, P.N. Britton); Montana State University, Bozeman, Montana, USA (R.K. Plowright); Griffith University Centre for Planetary Health and Food Security, Brisbane (A.J. Peel); University of Queensland School of Veterinary Science, Gatton, Queensland, Australia (A.C. Breed)
Main Article
Figure 4

Figure 4. Phylogenomics of novel HeV variant from horse in Australia. A) Maximum-likelihood phylogeny of paramyxoviruses using complete L protein sequences. Gray shading indicates henipaviruses, and red text indicates the novel HeV variant, which groups with the prototypic HeV. Bootstrap support values as proportions of 500 replicates are shown at nodes; values <0.7 are hidden. Scale bar indicates substitutions per site. B) Enlarged gray area from panel A shows branch lengths for the henipavirus clade. The branch leading back to the common ancestor of all known HeVs and the novel HeV variant does not exceed 0.03; thus, they are considered variants of the same species. C) Maximum-likelihood phylogeny of partial N and P where deep branch lengths have been collapsed for visualization only to demonstrate that the variant is well outside the known diversity of HeV. Scale bar indicates substitutions per site. D) Nucleotide genomic similarity of the variant compared with the prototypic HeV strain. V,W, and C indicate variably transcribed nonstructural proteins. F, fusion; G, glycoprotein; HeV, Hendra virus; L, paramyxovirus polymerase; M, matrix protein; N, nucleoprotein; P, phosphoprotein.
Main Article
Page created: December 30, 2021
Page updated: February 21, 2022
Page reviewed: February 21, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
