Volume 28, Number 4—April 2022
Research
Diminishing Immune Responses against Variants of Concern in Dialysis Patients 4 Months after SARS-CoV-2 mRNA Vaccination
Figure 1
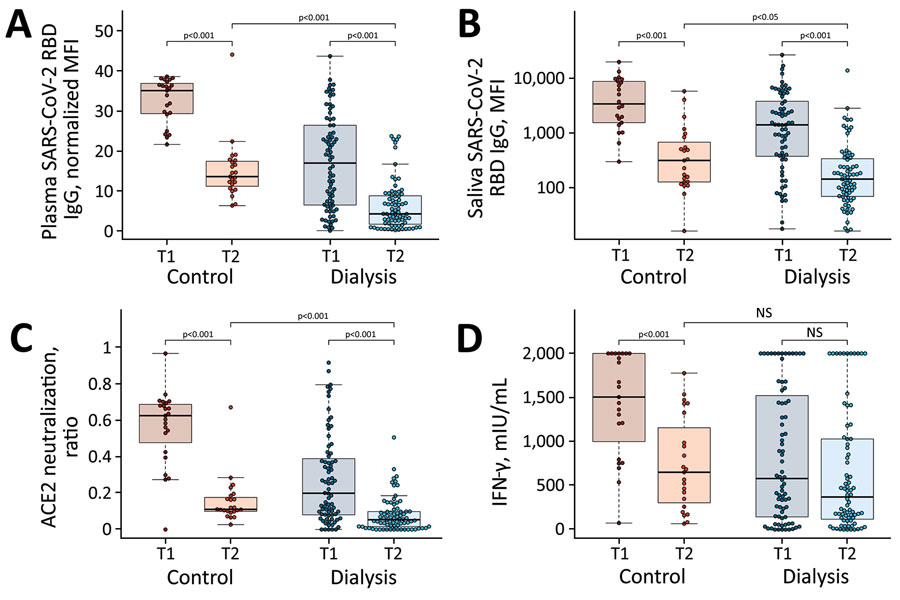
Figure 1. Significant decrease in humoral and cellular responses induced by Pfizer-BioNTech vaccine BNT162b2 (https://www.pfizer.com) against SARS-CoV-2 from 3 weeks to 16 weeks after second vaccination, observed in a study of immune response against variants of concern in dialysis patients 4 months after SARS-CoV-2 mRNA vaccination. A) IgG response in plasma; B) IgG response in saliva; C) neutralizing capacity toward SARS-CoV-2 wild type B.1; D) T-cell response measured by IFN-γ release assay. Blue circles indicate dialysis patients (n = 76) and red circles controls (n = 23). Samples were taken 3 weeks (T1) and 16 weeks (T2) after vaccination. Saliva (panel B) has reduced sample numbers in both groups because of issues in sample collection (T1 control, n = 22; T1 dialysis, n = 69; T2 control, n = 23; T2 dialysis. n = 71). T1 timepoint data has been published previously (13) and is reproduced here for clarity. Horizontal lines within boxes indicate medians; box tops and bottoms indicate the 25th and 75th percentiles; whiskers show the largest and smallest nonoutlier values. Outliers were determined by 1.5 times interquartile range. Statistical significance was calculated by Wilcoxon matched-pairs signed rank test when comparing T1 and T, and 2-sided Mann–Whitney–U test when comparing control and dialysis groups. ACE2, angiotensin-converting enzyme 2; IFN-γ, interferon γ; MFI; median fluorescence intensity; NS, not significant; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; T1, timepoint 1; T2, timepoint 2.
References
- European Centre for Disease Control and Prevention. Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA. 2021 [cited 2021 Aug 18]. https://www.ecdc.europa.eu/en/publications-data/overview-implementation-covid-19-vaccination-strategies-and-deployment-plans
- Haab BB. Methods and applications of antibody microarrays in cancer research. Proteomics. 2003;3:2116–22. DOIPubMedGoogle Scholar
- Ibarrondo FJ, Hofmann C, Fulcher JA, Goodman-Meza D, Mu W, Hausner MA, et al. Primary, recall, and decay kinetics of SARS-CoV-2 vaccine antibody responses. ACS Nano. 2021;15:11180–91. DOIPubMedGoogle Scholar
- Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001 Clinical Trial Group. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–73. DOIPubMedGoogle Scholar
- Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al.; mRNA-1273 Study Group. mRNA-1273 Study Group. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–61. DOIPubMedGoogle Scholar
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–2. DOIPubMedGoogle Scholar
- Altmann DM, Boyton RJ, Beale R. Immunity to SARS-CoV-2 variants of concern. Science. 2021;371:1103–4. DOIPubMedGoogle Scholar
- Heyse S, Vogel H, Sänger M, Sigrist H. Covalent attachment of functionalized lipid bilayers to planar waveguides for measuring protein binding to biomimetic membranes. Protein Sci. 1995;4:2532–44. DOIPubMedGoogle Scholar
- Girndt M, Trocchi P, Scheidt-Nave C, Markau S, Stang A. The Prevalence of Renal Failure. Results from the German Health Interview and Examination Survey for Adults, 2008-2011 (DEGS1). Dtsch Arztebl Int. 2016;113:85–91.PubMedGoogle Scholar
- Windpessl M, Bruchfeld A, Anders HJ, Kramer H, Waldman M, Renia L, et al. COVID-19 vaccines and kidney disease. Nat Rev Nephrol. 2021;17:291–3. DOIPubMedGoogle Scholar
- Schrezenmeier E, Bergfeld L, Hillus D, Lippert J-D, Weber U, Tober-Lau P, et al. Immunogenicity of COVID-19 tozinameran vaccination in patients on chronic dialysis. Front Immunol. 2021;12:
690698 . DOIPubMedGoogle Scholar - Carr EJ, Wu M, Harvey R, Wall EC, Kelly G, Hussain S, et al.; Haemodialysis COVID-19 consortium; Crick COVID Immunity Pipeline. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet. 2021;398:1038–41. DOIPubMedGoogle Scholar
- Strengert M, Becker M, Ramos GM, Dulovic A, Gruber J, Juengling J, et al. Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on haemodialysis. EBioMedicine. 2021;70:
103524 . DOIPubMedGoogle Scholar - Becker M, Strengert M, Junker D, Kaiser PD, Kerrinnes T, Traenkle B, et al. Exploring beyond clinical routine SARS-CoV-2 serology using MultiCoV-Ab to evaluate endemic coronavirus cross-reactivity. Nat Commun. 2021;12:1152. DOIPubMedGoogle Scholar
- Becker M, Dulovic A, Junker D, Ruetalo N, Kaiser PD, Pinilla YT, et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat Commun. 2021;12:3109. DOIPubMedGoogle Scholar
- Stankov MV, Cossmann A, Bonifacius A, Dopfer-Jablonka A, Ramos GM, Gödecke N, et al. Humoral and cellular immune responses against severe acute respiratory syndrome coronavirus 2 variants and human coronaviruses after single BNT162b2 vaccination. Clin Infect Dis. 2021;73:2000–8. DOIPubMedGoogle Scholar
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. DOIPubMedGoogle Scholar
- Lopez E, Haycroft ER, Adair A, Mordant FL, O’Neill MT, Pymm P, et al. Simultaneous evaluation of antibodies that inhibit SARS-CoV-2 variants via multiplex assay. JCI Insight. 2021;6:
e150012 . DOIPubMedGoogle Scholar - Ng JH, Ilag LL. Biochips beyond DNA: technologies and applications. Biotechnol Annu Rev. 2003;9:1–149. DOIPubMedGoogle Scholar
- Hartzell S, Bin S, Cantarelli C, Haverly M, Manrique J, Angeletti A, et al. Kidney failure associates with T cell exhaustion and imbalanced follicular helper T cells. Front Immunol. 2020;11:
583702 . DOIPubMedGoogle Scholar - Garcia P, Anand S, Han J, Montez-Rath ME, Sun S, Shang T, et al. COVID-19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol. 2022;33:33–7. DOIPubMedGoogle Scholar
- Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100:702–4. DOIPubMedGoogle Scholar
- Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–6. DOIPubMedGoogle Scholar
- Massa F, Cremoni M, Gérard A, Grabsi H, Rogier L, Blois M, et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine. 2021;73:
103679 . DOIPubMedGoogle Scholar - Stervbo U, Blazquez-Navarro A, Blanco EV, Safi L, Meister TL, Paniskaki K, et al. Improved cellular and humoral immunity upon a second BNT162b2 and mRNA-1273 boost in prime-boost vaccination no/low responders with end-stage renal disease. Kidney Int. 2021;100:1335–7. DOIPubMedGoogle Scholar
1These authors contributed equally to this article.
2These authors contributed equally to this article.