Durability of Antibody Response and Frequency of SARS-CoV-2 Infection 6 Months after COVID-19 Vaccination in Healthcare Workers
Eric D. Laing, Carol D. Weiss, Emily C. Samuels, Si’Ana A. Coggins, Wei Wang, Richard Wang, Russell Vassell, Spencer L. Sterling, Marana S. Tso, Tonia Conner, Emilie Goguet, Matthew Moser, Belinda M. Jackson-Thompson, Luca Illinik, Julian Davies, Orlando Ortega, Edward Parmelee, Monique Hollis-Perry, Santina E. Maiolatesi, Gregory Wang, Kathleen F. Ramsey, Anatalio E. Reyes, Yolanda Alcorta, Mimi A. Wong, Alyssa R. Lindrose, Christopher A. Duplessis, David R. Tribble, Allison M.W. Malloy, Timothy H. Burgess, Simon D. Pollett, Cara H. Olsen, Christopher C. Broder, and Edward Mitre
Author affiliations: Uniformed Services University, Bethesda, Maryland, USA (E.D. Laing, E.C. Samuels, S.A. Coggins, S.L. Sterling, M.S. Tso, T. Conner, E. Goguet, M. Moser, B.M. Jackson-Thompson, L. Illinik, J. Davies, O. Ortega, E. Parmelee, A.R. Lindrose, D.R. Tribble, A.M.W. Malloy, T.H. Burgess, S.D. Pollett, C.H. Olsen, C.C. Broder, E. Mitre); US Food and Drug Administration, Silver Spring, Maryland, USA (C.D. Weiss, W. Wang, R. Wang, R. Vassell); Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda (E.C. Samuels, S.A. Coggins, S.L. Sterling, E. Goguet, M. Moser, B.M. Jackson-Thompson, L. Illinik, J. Davies, O. Ortega, E. Parmelee, S.E. Maiolatesi, A.R. Lindrose, S.D. Pollett); Naval Medical Research Center, Silver Spring (L. Illinik, J. Davies, O. Ortega, E. Parmelee, M. Hollis-Perry, S.E. Maiolatesi, G. Wang, K.F. Ramsey, A.E. Reyes, Y. Alcorta, M.A. Wong, C.A. Duplessis); General Dynamics Information Technology, Falls Church, Virginia, USA (G. Wang, K.F. Ramsey, A.E. Reyes, Y. Alcorta, M.A. Wong)
Main Article
Figure 1
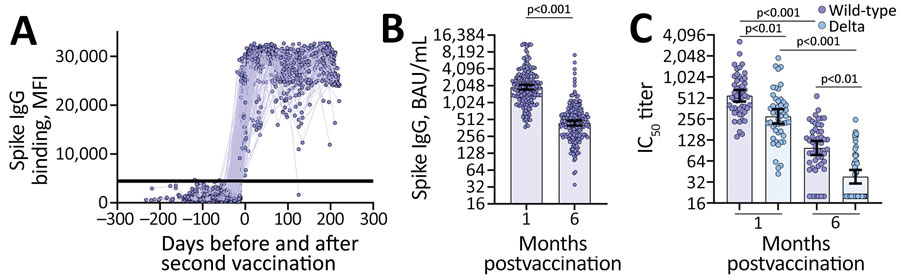
Figure 1. Vaccine-induced binding and neutralizing antibody responses observed among US healthcare worker participants in the Prospective Assessment of SARS-CoV-2 Seroconversion (PASS) study, January–August 2021. A) MFI levels of vaccine-induced spike IgG binding before and after second vaccination in serum samples diluted 1:400 (n = 227 participants). Horizontal line indicates the positive or negative spike IgG threshold. B) Spike IgG binding antibodies (BAU/mL) quantified from serum samples collected 1 month (mean 36.9 days, range 23–81 days) and 6 months (mean 201.1 days, range 151–237 days) postvaccination (n = 187 participants). Wilcoxon matched-pairs signed rank test performed; y-axis is log2-scale. C) Neutralizing antibody titers against severe acute respiratory syndrome coronavirus 2 wild-type and Delta variant from serum samples collected 1 month (mean 30.8 days, range 28–42 days) and 6 months (mean 200.1 days, range 189–219 days) postvaccination (n = 49 participants). Friedman ANOVA with Dunn’s multiple comparisons performed post-hoc; y-axis is log2-scale. All errors bars represent the geometric mean and 95% CIs. BAU, binding antibody units; IC50, 50% inhibitory concentration; MFI, median fluorescence intensity.
Main Article
Page created: January 25, 2022
Page updated: March 19, 2022
Page reviewed: March 19, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
