Volume 28, Number 5—May 2022
Dispatch
Evidence of Prolonged Crimean-Congo Hemorrhagic Fever Virus Endemicity by Retrospective Serosurvey, Eastern Spain
Abstract
We conducted a retrospective serosurvey for antibodies against Crimean-Congo hemorrhagic fever virus in wild ungulates along the eastern Mediterranean Coast of Spain. The virus has been endemic in this region since 2010 but is mainly restricted to geographic clusters with extremely high seropositivity associated with high density of bovids.
Crimean-Congo hemorrhagic fever (CCHF) is caused by CCHF virus (CCHFV), a tickborne pathogen of the genus Orthonairovirus, belonging to the family Bunyaviridae. In humans, CCHFV can induce a severe and potentially fatal systemic hemorrhagic disease. CCHFV infections in wildlife and domestic animals are generally subclinical but, in some species, can induce enough viremia to enable virus transmission to uninfected ticks. Moreover, infected animals produce antibodies, enabling the identification of affected areas through retrospective serologic studies (1). CCHFV is endemic in several countries in Asia, Africa, the Middle East, and southeastern Europe and has a range similar to that of its main vectors and reservoirs, Hyalomma spp. ticks, which are expanding their habitat range in southern Europe (2).
In Spain, CCHFV was detected in H. lusitanicum ticks from a red deer (Cervus elaphus) in 2010 (3). Since 2013, several severe CCHF cases in humans have been reported in the country (4). Viral strains identified in Spain showed high genetic variability, suggesting repeated introductions from different origins, including Africa and eastern Europe (5,6). Seroprevalence studies conducted in 2017 and 2018 showed evidence that CCHFV is prevalent over large areas of central and southern Spain, which coincide with the regions where H. marginatum and H. lusitanicum ticks have been described (4,6). Along the Mediterranean Coast of eastern Spain, the existence of CCHFV vectors (Hyalomma ticks) and of the virus itself were uncertain until recently, when H. lusitanicum ticks were found in wild boars (Sus scrofa) from the metropolitan area of Barcelona (7), and CCHFV seropositivity was reported in ungulates from southern Catalonia (8). To evaluate the extent and duration of CCHFV circulation in eastern Spain, we conducted a retrospective serosurvey to detect CCHFV antibodies in different wildlife species in the Valencia region.
We used the CCHF Double Antigen Multi-Species ELISA kit (IDvet, https://www.id-vet.com) to test for CCHFV antibodies in serum samples collected from 332 wild boars, 126 Iberian ibexes (Capra pyrenaica), and 48 mouflons (Ovis aries musimon). Serum samples were collected during 2010–2021 within the framework of the wildlife surveillance program in the Valencia region. We chose wild boars, Iberian ibexes, and mouflons because they are the main wild ungulate species in the region. Iberian ibexes and mouflons were selected from the 2 areas where they are more abundant. We also selected serum samples taken from boars in the same 2 areas and from areas with low densities of wild ruminants. (Appendix Figure 1).
Our results showed that CCHFV was already circulating in different areas of the Valencia region by the time the virus was reported in Spain in 2010 (Table; Appendix Figure 2). These results are consistent with the phylogenetic analysis of the CCHFV strain obtained from a H. lusitanicum tick collected in western Spain in 2014 that suggested the strain had been circulating in the country for several decades (9). Together with the variability of CCHFV strains identified in Spain (5,6), our findings suggest an epidemiologic scenario in which CCHFV has been repeatedly introduced into different regions of Spain over many years.
Among Iberian ibex serum samples from Valencia, 96.0% (121/126) had antibodies against CCHFV, which is close to the 100% seroprevalence reported for the same species in the affected neighboring area of Catalonia (8). Likewise, all the mouflon (48/48) samples in this study were seropositive, indicating a high susceptibility in this species, even though CCHFV infection has not been previously described in mouflons. In contrast, only 15.5% (51/332) of the wild boar samples tested were seropositive, and wild boars in the areas of high densities of Iberian ibexes and mouflons had seroprevalences of only 36.0% (49/136), which coincides with the results obtained in Catalonia (8). One possible explanation for the prevalences we found in Iberian ibexes, mouflons, and wild boars is that Hyalomma genus ticks feed preferably on species of the family Bovidae but also feed, although less prominently, in the family Suidae (10).
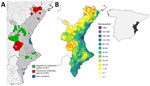
CCHFV seropositivity in the Valencia region clustered in 2 areas (Figure, panel A). One cluster was in the north in the Tinença de Benifassà Natural Park, an area of the region that is a continuation of the Ports de Tortosa-Beseit National Game Reserve, the affected area in Catalonia that is close to the Ebro Delta wetland (8). The other cluster was located at the Muela de Cortes y el Caroche natural area in central Valencia region, <40 km from the Albufera, the third-largest wetland in Spain. Identifying 2 main CCHFV transmission areas close to key stopover areas for migratory birds adds weight to the hypothesis of CCHFV introduction in Spain via migratory birds carrying infected ticks. In fact, the Mediterranean/Black Sea Flyway and the East Atlantic Flyway, 2 of the 3 Palaearctic-African flyways connecting Europe with Africa, converge on the Mediterranean Coast of eastern Spain.
We also detected CCHFV antibodies in a few wild boars outside the 2 main positive areas (Figure, panel A). Because wild boars are known to disperse over long distances (11), this species could play a key role in the spread of CCHFV outside endemic areas.
A recent study mapped the risk for CCHFV exposure among humans in mainland Spain by using red deer as an indicator of the transmission risk plus environmental variables (12), but that study did not predict areas of high risk that we identified in the Valencia region or those identified farther north (8). Those findings indicate that determinants of CCHFV circulation in central and southwestern Spain are clearly different from those in the Mediterranean area, where Iberian ibexes, and to a lesser extent wild boars and mouflons, likely play a key role.
Little information is available on the distribution of competent CCHFV vectors in the Valencia region, but a study to the north of the region reported a substantial increase during 2017–2018 in the number of persons receiving tick bites, 85% of which were caused by H. lusitanicum ticks (13). Other studies have suggested that the lack of human CCHF cases in the Mediterranean region, despite areas with widespread CCHFV, is the result of a low rate of contact between humans and infected ticks (14). At least in the Valencia region, this low contact seems to be the case; areas where CCHFV transmission in wildlife is concentrated coincide with the areas with the lowest human density (Figure, panel B). However, rising wild ungulate populations that are moving closer to densely populated areas could change the human epidemiologic situation.
Our results support an epidemiologic scenario in which CCHFV has been endemic in wild ungulates in different regions of Spain before it was detected in 2010. In eastern Spain, CCHFV circulation mainly occurs in geographic clusters associated with high densities of Bovidae species. However, as these species move into areas with higher human populations, more human CCHF cases could occur. To protect the population of the region, public health authorities should continue CCHFV surveillance among tick and ungulate species.
Ms. Carrera-Faja is a PhD candidate at the Wildlife Conservation Medicine Research Group (WildCoM), Department of Animal Medicine and Surgery, Universitat Autònoma, Barcelona, Spain. Her current research focuses on Crimean-Congo hemorrhagic fever virus in wildlife species.
References
- Spengler JR, Bergeron É, Rollin PE. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl Trop Dis. 2016;10:
e0004210 . DOIPubMedGoogle Scholar - Messina JP, Pigott DM, Golding N, Duda KA, Brownstein JS, Weiss DJ, et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg. 2015;109:503–13. DOIPubMedGoogle Scholar
- Estrada-Peña A, Palomar AM, Santibáñez P, Sánchez N, Habela MA, Portillo A, et al. Crimean-Congo hemorrhagic fever virus in ticks, Southwestern Europe, 2010. Emerg Infect Dis. 2012;18:179–80. DOIPubMedGoogle Scholar
- Ministry of Health. Consumption and Social Welfare. Report on the situation and evaluation of the risk of transmission of the Crimean-Congo hemorrhagic fever virus in Spain, July 2019 [in Spanish] [cited 2021 Nov 9]. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/analisisituacion/ doc/ER_FHCC.pdf
- Ramírez de Arellano E, Hernández L, Goyanes MJ, Arsuaga M, Cruz AF, Negredo A, et al. Phylogenetic characterization of Crimean-Congo hemorrhagic fever virus, Spain. Emerg Infect Dis. 2017;23:2078–80. DOIPubMedGoogle Scholar
- Moraga-Fernández A, Ruiz-Fons F, Habela MA, Royo-Hernández L, Calero-Bernal R, Gortazar C, et al. Detection of new Crimean-Congo haemorrhagic fever virus genotypes in ticks feeding on deer and wild boar, Spain. Transbound Emerg Dis. 2021;68:993–1000. DOIPubMedGoogle Scholar
- Castillo-Contreras R, Magen L, Birtles R, Varela-Castro L, Hall JL, Conejero C, et al. Ticks on wild boar in the metropolitan area of Barcelona (Spain) are infected with spotted fever group rickettsiae. Transbound Emerg Dis. 2021;
tbed.14268 ; Epub ahead of print. DOIPubMedGoogle Scholar - Espunyes J, Cabezón O, Pailler-García L, Dias-Alves A, Lobato-Bailón L, Marco I, et al. Hotspot of Crimean-Congo hemorrhagic fever virus seropositivity in wildlife, Northeastern Spain. Emerg Infect Dis. 2021;27:2480–4. DOIPubMedGoogle Scholar
- Cajimat MNB, Rodriguez SE, Schuster IUE, Swetnam DM, Ksiazek TG, Habela MA, et al. Genomic characterization of Crimean-Congo hemorrhagic fever virus in Hyalomma tick from Spain, 2014. Vector Borne Zoonotic Dis. 2017;17:714–9. DOIPubMedGoogle Scholar
- Spengler JR, Estrada-Peña A. Host preferences support the prominent role of Hyalomma ticks in the ecology of Crimean-Congo hemorrhagic fever. PLoS Negl Trop Dis. 2018;12:
e0006248 . DOIPubMedGoogle Scholar - Casas-Díaz E, Closa-Sebastià F, Peris A, Torrentó J, Casanovas R, Marco I, et al. Dispersal record of wild boar (Sus scrofa) in northeast Spain: implications for implementing disease-monitoring programs. Wildl Biol Pract. 2013;9:19–26. DOIGoogle Scholar
- Cuadrado-Matías R, Cardoso B, Sas MA, García-Bocanegra I, Schuster I, González-Barrio D, et al. Red deer reveal spatial risks of Crimean-Congo haemorrhagic fever virus infection. Transbound Emerg Dis. 2021;
tbed.14385 ; Epub ahead of print. DOIPubMedGoogle Scholar - Falcó Garí JV, López-Peña D, de la Torre J, Safont-Adsuara L, Bellido-Blasco J, Jiménez-Peydró R. Incidence of disease-transmitting ticks on the human population in the province of Castellón [In Spanish]. Rev Salud Ambient. 2019;19(Espec. Congr):135. Presented at: XV Environmental Health Congress; May 22–24, 2019; Valencia, Spain.
- Estrada-Peña A, de la Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 2014;108:104–28. DOIPubMedGoogle Scholar
- National Air and Space Administration. Socioeconomic data and applications center (SEDAC): gridded population of the world, version 4 (GPWv4): administrative unit center points with population estimates [cited 2021 Nov 3].
Figure
Table
Cite This ArticleOriginal Publication Date: April 07, 2022
1These senior authors contributed equally to this article.
Table of Contents – Volume 28, Number 5—May 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Johan Espunyes; Wildlife Conservation Medicine Research Group (WildCoM), Departament de Medicina i Cirurgia Animals, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain
Top