Volume 28, Number 5—May 2022
Research
Effectiveness of BNT162b2 Vaccine Booster against SARS-CoV-2 Infection and Breakthrough Complications, Israel
Figure 1
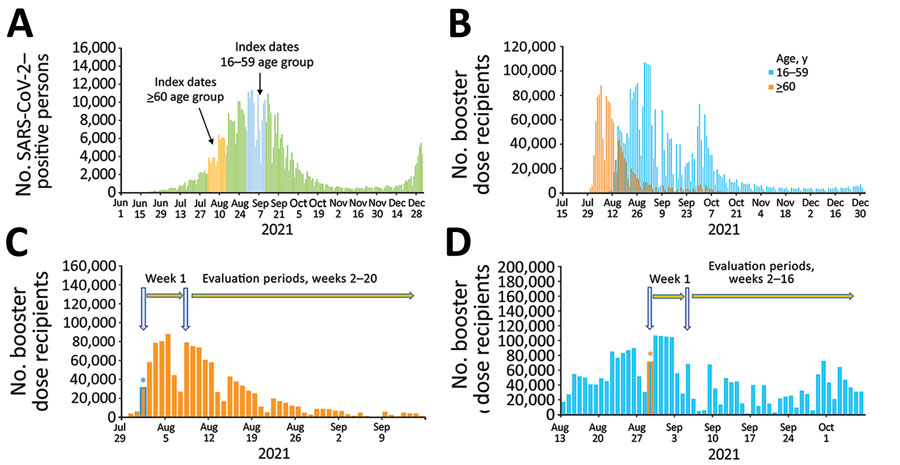
Figure 1. Estimations of effectiveness of BNT162b2 vaccine booster (Pfizer, https://www.pfizer.com) against SARS-CoV-2 infection and breakthrough complications, Israel. A) Epidemic curve of new PCR-confirmed SARS-CoV-2–positive persons, June 1, 2021–January 1, 2022. Index dates are highlighted in orange (for persons >60 years of age) and light blue (for persons 16–59 years of age). B) Daily booster dose recipients by age group. C) Graphic illustration of the booster dose vaccine effectiveness evaluation method for a single cohort of persons >60 years of age that received the booster dose on August 1, 2021. Orange bars represent the number of persons who received the booster dose each day; light blue asterisk represents the date persons >60 years of age included in cohort 1 received the booster dose. D) Graphic illustration of the booster dose vaccine effectiveness evaluation method for a single cohort of persons 16–59 years of age who received the booster dose on August 29, 2021. Light blue bars represent the number of persons who received the booster dose each day; orange asterisk represents the date persons 16–59 years of age included in cohort 1 received the booster dose.